Anticoagulation During AF Ablation: The Balance between Thromboembolism and Bleeding
Jennifer A. Mears, BS1, Samuel J. Asirvatham, MD1,2
1Division of Cardiovascular Diseases, Department of Internal Medicine.2Department of Pediatrics and Adolescent Medicine Mayo Clinic, Rochester, Minnesota.
Radiofrequency ablation for atrial fibrillation is being increasingly used to treat patients with symptomatic arrhythmia. The procedure is complex and associated with significant complications including thromboembolism, stroke, and bleeding. Despite significant advances in catheter design, online cardiac imaging, and greater operator experience, both stroke and major vascular complications continue to be problematic. Increasing the duration and intensity of anticoagulation has been the primary modality used to decrease thromboembolism. However, these measures increase the likelihood and severity of bleeding-related complications. The optimal method of anticoagulation along with the adjunctive use of technology to decrease vascular complications and mechanically prevent cerebral embolization is unknown. In this paper, we review the present methods used by ablationists to decrease the likelihood of thromboembolism during atrial fibrillation. We then describe methods used to decrease bleeding and vascular complications at access sites as well as cardiac perforation. We briefly discuss newer techniques to decrease endovascular complications including epicardial ablation and the use of temporarily implanted vascular protection devices.Finally, we describe the best option or combination of approaches that attempt to balance the risks of thromboembolism and bleeding during AF ablation..
Correspondence to: Samuel J. Asirvatham, M.D.,Division of Cardiovascular Diseases,Associate Professor of Medicine,Mayo Clinic College of Medicine,200 First Street SW,Rochester, MN 55905.
Over the last decade, radiofrequency (RF) ablation for atrial fibrillation (AF) has emerged as an increasingly used and effective technique to manage patients who are symptomatic from this arrhythmia. There has been a literal explosion in both the clinical utilization of ablation and research attempting to identify the best targets and the safest methods to invasively treat AF.1-4 Given how common AF is, affecting more than 2 million people in the United State alone, and the fact that it is reasonably well tolerated, make it important that ablation be as safe as possible to recommend this treatment modality.5
Of the several complications that may occur during AF ablation, stroke, cardiac perforation, and major bleeding are among the most feared. While any invasive cardiac procedure may be associated with perforation or bleeding and any left-sided procedure may result in thromboembolism and stroke, AF ablation particularly highlights these issues. Regardless of the approach used for endocardial AF ablation, extensive RF applications in the left atrium are the rule, simultaneously, increasing the risk of thrombus formation and cardiac perforation. Further, patients with chronic AF tend to have larger left atria, are older, are often on preprocedure anticoagulation, and require postablation intensive anticoagulation, thus disproportionately at risk for both thrombus and bleeding.6-8
In this review, we describe the present methods used by ablationists to try and minimize thromboembolic risk during ablation. We subsequently review techniques to decrease bleeding and vascular complications as well as newer methods to avoid endovascular complications including epicardial ablation and the use of temporarily inserted vascular protection devices. While reviewing the existing literature, we evaluate these techniques in terms of which combinations achieve the balance between thromboembolism and bleeding during AF ablation procedures.
Thromboembolism as a Complication
of AF Ablation
Ablationists rightfully try to avoid any RF ablation procedure-related complication. However, stroke as a periprocedural complication evokes particular dread because the event and its sequelae are so devastating, and we lack a clear understanding of how to prevent it.7,9 While advances in remote/robotic catheter navigation along with better online imaging techniques, experience with safe catheter manipulation, and knowledge and ability to recognize appropriate RF ablation targets may decrease other complications, they do not necessarily impact the risk of thrombus formation. The pathogenesis of thrombus and subsequent embolization during left atrial ablation is multifactorial.10 During transseptal puncture, the endothelial denudation that occurs may be thrombogenic. Simply placing sheaths or catheters in the circulation may be sufficient to give rise to soft thrombus. Most importantly, however, during RF energy delivery, the associated local temperature rise can result in coagulum formation that in turn can be a nidus for propagating soft thrombus.8,11 Periprocedural heparinization has been the mainstay for interventionalists to decrease this complication. We review approaches that involve increased intensity, duration, or location for heparinization and describe the limitations of this pharmacological agent in impacting the occurrence of coagulum during ablation. These limitations are responsible for the unfortunate statistic that despite adequate heparinization, about 2% of complex left-sided ablation procedures continue to be associated with thromboembolism.12,13
Bleeding and Vascular Complications During AF Ablation
With the multiple catheters and sheaths frequently required for complex AF ablation, access site bleeding and various vascular complications including pseudoaneurysm formation and retroperitoneal bleeding are more common with these procedures. Further, cardiac perforation is a potentially fatal occurrence and occurs in about 2.4% of patients undergoing AF ablation.14 While pericardiocentesis and possible placement of indwelling drains or open-chest surgical closure of the perforation may prevent catastrophe, these bleeding risks significantly impact outcomes.14 While anticoagulation at least in part mitigates against major thromboembolism, there is an inherent increase in the risk for bleeding and vascular complications. Each approach to minimize thromboembolic risk must be evaluated not only in terms of its own efficacy but also against the extent of the propensity to increase bleeding.
Approaches to Minimize Thromboembolic Rrisk
Increasing the Intensity of Anticoagulation
The most straightforward approaches to decrease thromboembolism are to simply increase the intensity of anticoagulation with intravenous heparin. In fact, studies have demonstrated that high intensity anticoagulation, once vascular access has been obtained, can be performed without undue bleeding risk.15,16 Ren and coworkers studied 511 patients undergoing pulmonary vein isolation for AF. Patients were divided into two groups based on the intensity of heparinization. The first group had a target ACT of 250-300 seconds while the second group had a target ACT of 300-410 seconds. Relatively high-risk patients for thromboembolism were included in this study as evidenced by the occurrence of spontaneous echo contrast (SEC) in 16.7% of patients in group 1 and 59.9% of patients in group 2. Interestingly, despite the higher prevalence of SEC in group 2 (suggesting a higher risk of thrombus formation), the overall occurrence of ultrasound-detected thrombus was significantly lower (2.8% vs 11.2% in group 1) with higher intensity anticoagulation. Importantly, there was no difference between the two groups in terms of procedural complications including bleeding, retroperitoneal hemorrhage, cardiac perforation, or other vascular complications. Although this study did include more than 500 patients, given the relatively low incidence of major thromboembolic or bleeding complications, it is difficult to know the clinical impact of high intensity anticoagulation in terms of stroke prevention and the risk of vascular complications, particularly with inexperienced operators. Further, the upper limit (is more always better?) for heparinization and targeted ACTs is not known, and presumably, at some point, bleeding risk will outweigh any incremental benefit in reducing thromboembolism.
Simply increasing the intensity of anticoagulation produces global effects including inhibition of coagulation at sites remote from the cardiac locations for ablation. This results in an increased risk of bleeding complications at sites where anticoagulation is not required at all. Some operators have attempted to locally enhance anticoagulation. Maleki et al reported one such technique and showed the reduction in the risk of intracardiac echocardiography (ICE)-detected thrombus formation.17 One-hundred-and-eighty patients who underwent ICE-guided transseptal catheterization were divided into two equal groups. In the first group, standard 2 U/cc concentration of heparin was used to flush the transseptal sheaths prior to left atrial access. In the second group, a much higher concentration of 1000 U/cc of heparin was used for the transseptal flush. They found a 1% incidence of thrombus formation detected by ICE in group II, which was significantly lower when compared to a 9% incidence noted in group I with the standard concentration of heparin flush. The authors, based on these observations, recommended higher concentration of heparin for any sheath placed in the left-sided circulation.17
It is not possible from this study to know whether bleeding risk is actually lower with targeted high intensity anticoagulation versus simply increasing the amount of heparin delivered intravenously. Further, thrombus formation does not occur exclusively around transseptal sheaths or within the lumen of such sheaths but rather can occur as a result of left atrial endothelial disruption from catheter manipulation or during the ablation process itself.
Timing of Anticoagulation
Because of the risk of cardiac perforation occurring specifically during transseptal puncture, heparin administration was routinely delayed until after the last (usually second) transseptal puncture was performed. With the use of intracardiac ultrasound, it became apparent that when the first transseptal puncture was performed, endothelial disruption and the introduction of this foreign body into the left atrium provided the milieu for rapid thrombus formation in the catheter lumen as well as the left atrium. As facility with dual transseptal puncture increased with operator experience, earlier heparinization either before the second transseptal or before transseptal puncture has been tried. Bruce and coworkers reported their findings in 508 patients who underwent AF ablation with ICE guidance.18 All patients received unfractionated heparin during the procedure, but the timing varied. In the first group of 31 patients, heparin was given immediately after vascular access was obtained and well prior to the first transseptal puncture. In the second group of 257 patients, heparinization was done after the first but before the second transseptal puncture. Finally, in the third group of 220 patients, heparinization was initiated only after the second transseptal puncture. ICE-detected thrombus was significantly lower in the group where the earliest heparinization occurred (0% group 1, 3.1% group 2, and 9% group 3 where anticoagulation was done only after the second transseptal puncture).Because of the risk of cardiac perforation occurring specifically during transseptal puncture, heparin administration was routinely delayed until after the last (usually second) transseptal puncture was performed. With the use of intracardiac ultrasound, it became apparent that when the first transseptal puncture was performed, endothelial disruption and the introduction of this foreign body into the left atrium provided the milieu for rapid thrombus formation in the catheter lumen as well as the left atrium.18-20 As facility with dual transseptal puncture increased with operator experience, earlier heparinization either before the second transseptal or before transseptal puncture has been tried. Bruce and coworkers reported their findings in 508 patients who underwent AF ablation with ICE guidance.18 All patients received unfractionated heparin during the procedure, but the timing varied. In the first group of 31 patients, heparin was given immediately after vascular access was obtained and well prior to the first transseptal puncture. In the second group of 257 patients, heparinization was done after the first but before the second transseptal puncture. Finally, in the third group of 220 patients, heparinization was initiated only after the second transseptal puncture. ICE-detected thrombus was significantly lower in the group where the earliest heparinization occurred (0% group 1, 3.1% group 2, and 9% group 3 where anticoagulation was done only after the second transseptal puncture).
It should be noted, however, that ICE was used in all patients in this study, and it is, therefore, unknown whether procedural risk would’ve been higher if fluoroscopy alone had been used for transseptal puncture in the patient group with early heparinization. Further, this study is longitudinal and nonrandomized. It is highly likely, therefore, that the later patients were the ones who received early heparinization and thus would correspond to the group of patients undergoing ablation by operators that were now more skilled and experienced with the procedure.
Uninterrupted Anticoagulation
A major problem of managing anticoagulation for patients undergoing AF ablation has been the need to discontinue warfarin prior to the procedure. In patients with an indication for oral anticoagulation with atrial fibrillation, most centers would discontinue this agent 3-4 days prior to the procedure. Typically, clinical judgment is used to identify patients who would need to be “bridged” with subcutaneous low-molecular-weight heparin or those in whom warfarin is simply discon tinued. The small but existing risk of thrombus formation during this warfarin-free hiatus needs consideration. More importantly, reinitiating warfarin following ablation can be problematic. In addition to the existing preprocedural patient risk for stroke following extensive left atrial ablation with large areas of endothelial denudation and isolated segments of the left atrium and pulmonary veins, there is a likely increase in stroke risk in the immediate postprocedural period. Thus, most centers will again “bridge” the patient with low-molecular-weight heparin until the INR is therapeutic. In the immediate postprocedural period, there is also an enhanced risk for bleeding and vascular complications, generally thought to be higher with boluses of heparin than with warfarin.6,15,21 Wazni et al reported a unique method of avoiding the need for a warfarin-free hiatus.22 They compared outcomes in three groups of patients with paroxysmal AF undergoing pulmonary vein isolation for AF. The first group received enoxaparin 1mg/kg twice daily for bridging following ablation, group 2 received enoxaparin 0.5 mg/kg twice daily, and group 3 had uninterrupted warfarin at the patient’s usual therapeutic dose. Many important results are to be noted. One patient in group 1 and 2 patients in group 2 developed stroke, but none in group 3 where warfarin had been continued. Hematoma formation was actually observed to occur less frequently in the patients where warfarin had been continued. This important finding that thromboembolic risk can be decreased without increasing the risk of bleeding by simply not interrupting oral anticoagulation represents a novel and previously untested approach. It should be noted that this study was performed in a very high volume ablation center involving skilled and experienced operators. Also noteworthy is that all patients in this study had paroxysmal AF. Since the number of RF energy deliveries and the required extent of complex catheter manipulation is typically more with chronic AF along with the likely increased chance for de novo thrombus to occur from stasis, the safety of uninterrupted anticoagulation is not known for these patients. Wazni et al also noted that SEC was more commonly observed in the patients where warfarin had been discontinued. This is a perplexing finding since warfarin does not decrease the propensity for erythrocytes to form rouleaux – the cause for SEC. The possibility exists that the ICE-detected SEC may in fact have demonstrated small thrombi which were disproportionately more frequently found in the patients that formed groups 1 and 2.6 Despite these possible limitations, the approach of simply continuing warfarin if validated in other patient populations and with less experienced operators solves many of these sticky issues associated with optimizing periprocedural anticoagulation.
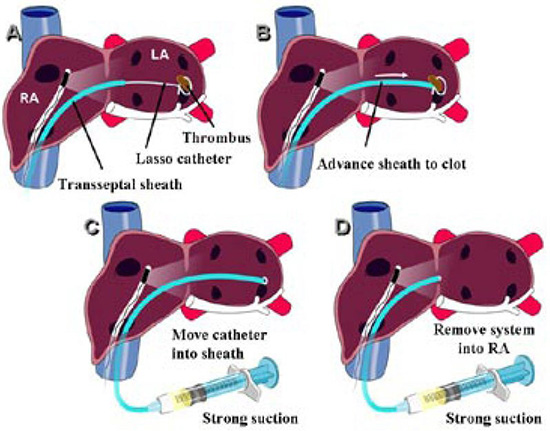
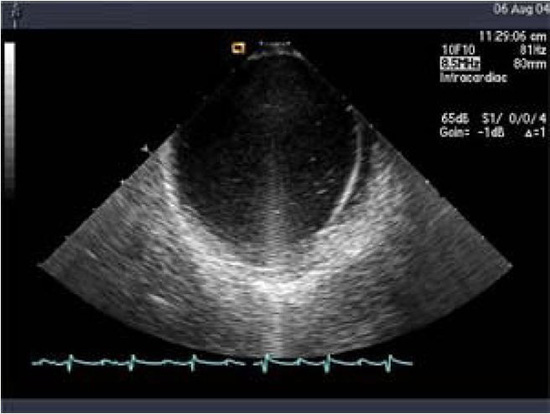
Linear phased-array intracardiac ultrasound imaging has been extensively used during AF ablation procedure23-29 (Figure 1). Although not directly a measure to prevent thromboembolism or bleeding, this imaging modality strongly impacts various other techniques that aim to minimize complications. ICE (Acuson, Siemens Medical Solutions, Malvern, PA) visualization is performed from the right atrium with an 8-10 Fr probe placed via the femoral veins. Imaging of the left atrium and pulmonary veins during ablation is used to recognize thrombus formation and possibly allow extraction of the thrombus when it occurs27 (Figure 2). Ren et al reported the incidence, risk factors, and outcome of managing left atrial thrombus with ICE.19 They studied 232 patients undergoing pulmonary vein isolation for AF. Intracardiac echocardiography detected thrombus in the left atrium in 24 of 232 patients (10%). These operators either immediately removed the thrombus from the left atrium with ICE guidance and suctioned up the thrombus through a sheath, or aggressive anticoagulation was performed (Figure 3). This study shows not only the ability of ICE imaging to identify thrombus but to potentially guide management of this complication once it occurs (Figure 4).
Figure 1. Intracardiac ultrasound using a linear phased-array probe placed in the right atrium visualizing the intraatrial septum and the left atrium. Note a strand-like structure consistent with thrombus noted in the left atrium despite adequate heparinization
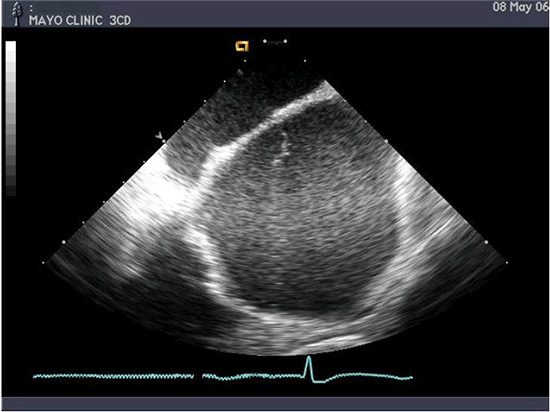
Figure 2. An ultrasound probe placed in the right atrium in the region of the interatrial septum which is used to visualize thrombus. Thrombus can be detected at various locations including on the transseptal sheath/Lasso catheter, ablation catheter or in the left atrium. Reproduced with permission from : Bruce CJ et al., J Interv Card Electrophysiol 2008; 22:211-219
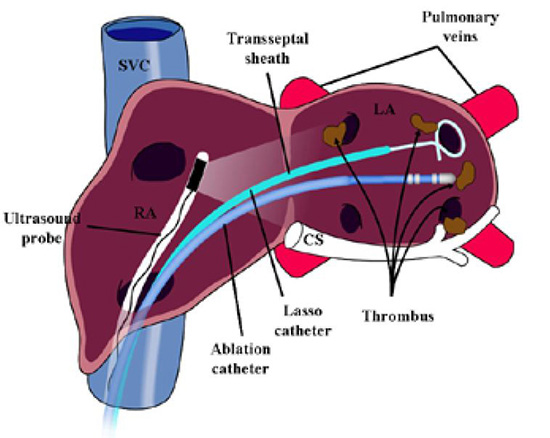
Figure 3. Panel A: Intracardiac ultrasound image during catheter ablation with ACT maintained above 300 seconds. A complex thrombus is noted in relationship to the circumferential mapping catheter. With appropriate placement of a vascular protection device and strong suction applied, the thrombus was removed in its entirety and is shown in Panel B. Reproduced with permission from : Bruce CJ et al., J Interv Card Electrophysiol 2008; 22:211-219
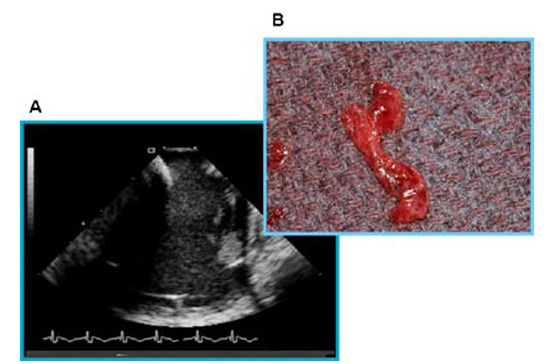
Figure 4. Technique for extraction of the ICE-detected thrombus: Panel A: An ICE probe is placed in the right atrium to visualize thrombus in the region of the Lasso catheter Panel B: The transseptal sheath is advanced close to the detected thrombus Panel C: Strong suction through the sidearm of the transseptal sheath is applied Panel D: While continued strong suction is being applied, the transseptal sheath is brought back to the right atrium while theLasso catheter is pulled into the transseptal sheath. Reproduced with permission from : Bruce CJ et al., J Interv Card Electrophysiol 2008; 22:211-219
There is also widespread utilization of ICE to guide transseptal puncture. This procedure has gone from being considered a “high risk for perforation” step in ablation to one that is now routinely and expeditiously performed with very low risk of perforation because of the extra imaging information, safety, and confidence that ICE has provided. Some approaches to decrease thromboembolism, for example early heparinization, may not have been considered but for ICE assistance with transseptal puncture particularly with inexperienced operators.30-32
Bleeding complications may also be quickly evaluated by ICE.14 When hypotension is noted, pericardial effusions can be quickly detected and managed, again giving operators more confidence to maximize anticoagulation. Similarly, if no effusion is noted, the operator may be alerted to the possibility of vascular bleeding including retroperitoneal hemorrhage.
Modifying Ablation Energy Delivery
Since increased temperatures resulting from ablation can trigger the coagulation cascade and directly denature fibrinogen into fibrin-coagulum, methods to modify ablation energy delivery have been tried to minimize the risk of thromboembolism.
Radiofrequency energy delivery does not directly heat the electrode but rather heating occurs at the tissue interface (Ohmic resistance site) which in turn heats the electrode. Since the thermistor or thermocouple used to monitor temperature is located at variable distances from the tissue interface within the distal electrode, an inherent discrepancy between measured temperature and tissue as well as interface heating exists. Therefore, very high temperatures may be occurring (greater than 85-100°C) even when the temperature “cutoff” is set much lower (55-65°C).11,33 The resultant clot formation may occur at the tissue surface or on the electrode. Efforts to minimize clot formation on the electrode surface have included power control ablation with careful monitoring of impedance and ICE visualization of “microbubbles”, a possible manifestation of excessive heating and the use of open saline irrigation (Figure 5).
Figure 5. Intracardiac ultrasound image of the left atrium and catheter during ablation energy delivery. Fine microbubbles are visualized. The use of microbubbles to titrate energy delivery has been described as one method of preventing impedance rise and coagulum formation (Oh S, et al. Avoiding microbubbles formation during radiofrequency left atrial ablation versus continuous microbubbles formation and standard radiofrequency ablation protocols: comparison of energy profiles and chronic lesion characteristics. J Cardiovasc Electrophysiol. 2006;17:72-7.)
Open saline irrigation of the electrode actively cools the electrode surface, preventing the high temperatures that can result in coagulum and subsequent thrombus formation on the electrode surface. In-vivo studies using the thigh muscle preparation muscle model34,35 have shown significant reduction of surface coagulum with open irrigation when compared to conventional catheters as well as internal saline irrigated electrodes.
Whether tissue thrombus formation is actually prevented as well or coagulum that does form is “washed away” is not presently clear. Dorwarth et al examined different catheter systems to identify which had the least risk of thrombus formation in excised pig hearts bathed with heparinized blood.36 Both external irrigation (showerheadtype electrode tips and a porous metal tip) as well as internally cooled catheters were studied. In addition, comparison was made with conventional catheters with a large surface electrode and standard catheters. In this study, the presence of coagulum was almost entirely abolished using the irrigation catheters. The authors also observed an overall lower rise in impedance with the use of external irrigation catheters. Internally cooled ablation catheters had a high coagulum formation rate when impedance rises were noted (38%). Large surface area catheters almost invariably showed thrombus when impedance rise was present.
These findings of likely decreased risk of thromboembolism with open saline irrigation must be balanced with the following: * Difficulty with monitoring and titrating ablation energy delivery as temperature monitoring is not valid. * Continued observation of coagulum on the proximal edge of the distal electrode where saline irrigation is not occurring * Possible increased incidence of myocardial disruption and perforation.
Other approaches have included the use of cryo-energy, which may possibly be associated with less risk of coagulum formation and the use of focused ultrasound energy, which has been conjectured to be associated with less endothelial disruption.24,37-40
Decreasing Available Fibrinogen during RF Energy Delivery
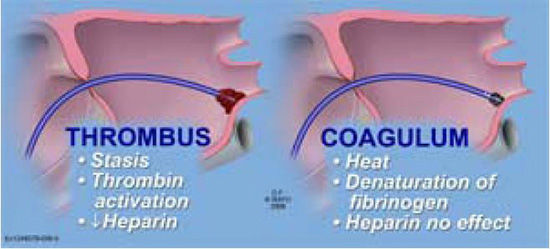
Imperative for any paradigm being successful in limiting thromboembolic complications is understanding the difference between thrombus and coagulum. Although these terms have been used interchangeably, thrombus formation is a thrombin-dependent process with subsequent change of fibrinogen to fibrin (clot). Coagulum formation specifically refers to the direct heat-related denaturation of fibrinogen to fibrin that is independent of thrombin. Heparin, which via antithrombin III inhibits thrombin and thus prevents clot formation, is completely ineffective in preventing coagulum development.10,41,42 As a result of this critical distinction, regardless of the intensity of anticoagulation with heparin, RF energy and the heat generated will still have the propensity to generate coagulum/char (Figure 6).
Figure 6. Illustration showing the difference between thrombus and coagulum. Coagulum results from direct heat denaturation of fibrinogen to fibrin. The process is thrombin independent and thus cannot be prevented by heparin. The coagulum itself may form a nidus for thrombus formation and subsequent obligation or embolization
Therefore, approaches have been suggested and are being investigated to minimize fibrinogen availability at the site of RF energy delivery. Rarely used but theoretically possible is to decrease fibrinogen levels using a fibrinogenolytic agent such as Ancrod.43,44 Because of the difficulty with monitoring drug effect and possible significant bleeding increase, such options are rarely used.
A novel approach being investigated is the simultaneous placement of direct current negative charge on catheter’s electrodes including while delivering RF energy. The negative charge intended to stabilize the fibrinogen molecule8,27,45 may repel the negatively-charged fibrinogen molecule, decreasing the availability of fibrinogen near the ablation electrode surface.
In addition, direct current application even at low levels (less than 100 μA) is associated with surface electrolysis producing a stream of small microbubbles. The generation of these electrolytic bubbles may act as a type of irrigation on the surface of electrodes again decreasing the amount of fibrinogen in contact with the electrode surface that can be denatured to fibrin with heating. Lim et al have reported ex-vivo and in-vitro observations where such surface application of negative direct current charge showed decreased quantity and thickness of coagulum formation on electrodes that had the current applied.8
Decreasing the Risk of Bleeding during
AF Ablation
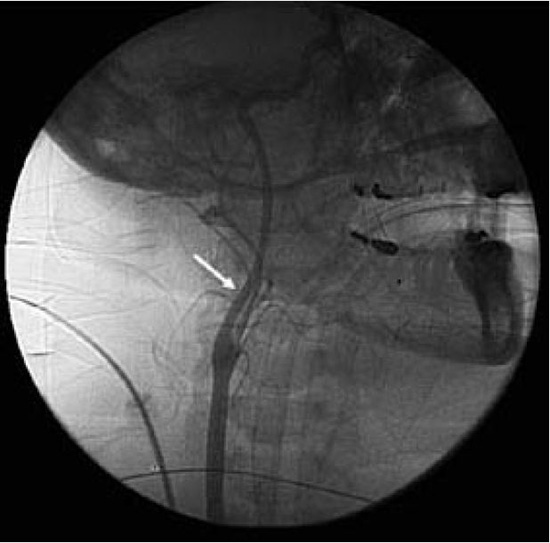
It is clear that some amount of anticoagulation is an absolute necessity during left atrial ablation, and although the optimal method of this anticoagulation is not known, with whatever modality is used, there is some decrease in the likelihood of stroke, and there is some increase in the risk of bleeding and vascular complications.6,12 Meticulous attention to technical details during vascular access and transseptal puncture can greatly decrease the chance of these complications despite high intensity anticoagulation. Should such complications arise, however, the risks of more serious consequences including pericardial tamponade or hemodynamically significant examination become relevant. Newer technical advances, including the use of intracardiac ultrasound described above, indirectly aim to decrease bleeding complications with more precise visualization of the intracardiac structures, catheter contact, etc. Similarly, devices to reduce the risk of excessive bleeding following femoral sheath removal have become widespread (Figure 7). Several mechanical methods to aid femoral arterial compression are available.46 Walker et al compared the use of the FemoStop® (RADI Medical Systems, Uppsala, Sweden) with manual compression in reducing femoral puncture site complications.46 Other studies have shown a wide variety of advantages with mechanical device compression, particularly when large French sheaths are introduced into the vasculature.47-50
Figure 7. Lateral fluoroscopic image showing carotid angiography and deployment of a temporary vascular protection device (arrow)
Another vascular closure device used is a percutaneous suture device. Kahn et al describe their experience with the Prostar-Plus (Perclose, Redwood City, CA), a suture-mediated arterial closure device.51,52 They use a guidewire to exchange the existing arterial sheath for an 8 Fr closure sheath. They utilized this device in over 10,000 diagnostic and interventional catheterizations. A significantly higher complication rate (major and minor) was noted in diagnostic catheterizations in patients treated with the Prostar-Plus device compared to manual compression (2.6% [major] and 4.1% [minor] complication rate for Prostar-Plus compared to 0.2% [major] and 1.8% [minor] with manual compression). No statistical differences were noted for major and minor complications in interventional catheterizations. However, there have been other studies that have contradicted Kahn and colleagues’ findings, making this suture-mediated closure device a consideration to enhance safety.53-55
Beyond Anticoagulation: Methods to
Decrease Thromboembolism
Since thromboembolism is an endovascular complication, ablation primarily performed on the epicardial surface of the heart would be associated with significantly less risk.56,57 Epicardial approaches for AF ablation have included pulmonary vein isolation, ablation in the Bachmann’s bundle region, and ablation of the retro-atrial ganglionated plexi.58 Epicardial approaches do have significant limitations, however, including difficulty with navigating catheters in patients with prior cardiac surgery, inadequate transmural lesion creation, and the inability to ablate common atrial flutter that may coexist with AF.
In addition, if transmural lesions are created, there is some endothelial disruption and thus potential for thromboembolism may still occur. A particularly difficult situation may arise when endothelial disruption or thrombus is recognized from epicardial ablation and intensive anticoagulation has been made problematic as a result of subxiphoid access and pericardial manipulation. The procedure of obtaining epicardial access may be associated with bleeding risk including hepatic hematoma formation and subdiaphragmatic hemorrhage.
Use of Vascular Protection Devices
A number of vascular protection devices designed to capture thromboembolic debris transiting from the heart towards the carotid circulation and brain have been developed and evaluated for their efficacy in reducing stroke59,60 (Figure 7). In one iteration, a temporary intravascular filter placed in the distal internal carotid artery during ablation or an intervention to capture thrombus is being attempted.59
These filters are miniature baskets placed over a guidewire and when delivered, are located in the carotid arteries. When completely expanded, the filters aim to capture any downstream debris, minimizing the risk of cerebrovascular events. Martelo et al59 reported a case of left atrial thrombus found during RF ablation by ICE. The thrombus was successfully retrieved by withdrawing the catheters with suction applied on the sheath while protecting the anterior cerebral circulation with a temporary carotid artery filter.59 The authors (SJA) now routinely deploy vascular protection devices into the carotid arteries when left atrial thrombus has been identified and further catheter manipulation either to withdraw the catheters or to retrieve clot is being attempted (Figure 3).
Balancing Thromboembolic and Bleeding
Risk
While there are many techniques that have been suggested to reduce the risk of thromboembolism during AF ablation, the most effective while simultaneously being associated with acceptable bleeding risk is not known. In our practice, once vascular access is obtained, high intensity anticoagulation with heparin administered both intravenously and as the flush through the transseptal sheaths is utilized. Intracardiac ultrasound is used to guide transseptal access and define catheter contact to avoid undue pressure and possible perforation. Open irrigation catheter use or monitoring impedance and microbubble formation when ablating with non-irrigated catheters are utilized to decrease electrode-related coagulum formation. Close attention to the technique for vascular access, use of ultrasound to identify the femoral arteries and veins, and the use of closure devices for arteries when larger French arterial sheaths are deployed help minimize bleeding. Other centers have preferred continuing oral anticoagulation or some combination of the above mentioned approaches.
There is an inherent conundrum in left atrial or ventricular ablation procedures where there is a simultaneous risk of thromboembolism along with the excess risk of bleeding as a result of anticoagulation meant to mitigate against thromboembolism. The judicious use and timing of anticoagulation along with careful vascular access technique and the use of intracardiac ultrasound may allow the best and safest results to be obtained.