The Arrhythmic Substrate for Atrial Fibrillation in Patients with Mitral Regurgitation
Matthew R. Schill1, Phillip S. Cuculich2, Christopher M. Andrews3, Ramya Vijayakumar3, Chawannuch Ruaengsri1, Matthew C. Henn1, Timothy S. Lancaster1, Spencer J. Melby1, Richard B. Schuessler13, Yoram Rudy23, Ralph J. Damiano1
1Department of Surgery, Division of Cardiothoracic Surgery, Washington University in St. Louis; 660 S. Euclid Ave., Campus Box 8234, St. Louis, MO 63110, USA.2Department of Medicine, Cardiovascular Division, Washington University in St. Louis; 660 S. Euclid Ave., Campus Box 8086, St. Louis, MO 63110, USA.3Department of Biomedical Engineering, Washington University in St. Louis; 1 Brookings Dr., Campus Box 1097, St. Louis MO 63130, USA.
Patients with severe mitral regurgitation commonly develop atrial fibrillation. The precise mechanisms of this relationship remain unknown. The objective of this study was to apply noninvasive electrocardiographic imaging of the atria during sinus rhythm to identify changes in atrial electrophysiology that may contribute to development of atrial fibrillation in patients with severe mitral regurgitation referred for mitral valve surgery.
Twenty subjects (9 atrial fibrillation and mitral regurgitation, 11 mitral regurgitation alone) underwent electrocardiographic imaging. Biatrial electrophysiology was imaged with activation maps in sinus rhythm. The reconstructed unipolar electrograms were analyzed for voltage amplitude, number of deflections and conduction heterogeneity. In subjects with mitral regurgitation, left atrial biopsies were obtained at the time of surgery. Results: Subjects with history of atrial fibrillation demonstrated prolonged left atrial conduction times (110±25 ms vs. mitral regurgitation alone (85±21), p=0.025); right atrial conduction times were unaffected. Variable patterns of conduction slowing were imaged in the left atria of most subjects, but those with prior history of atrial fibrillation had more complex patterns of conduction slowing or unidirectional block. The presence of atrial fibrillation was not associated with the extent of fibrosis in atrial biopsies.
Detailed changes in sinus rhythm atrial electrophysiology can be imaged noninvasively and can be used to assess the impact and evolution of atrial fibrillation on atrial conduction properties in patients with mitral regurgitation. If replicated in larger studies, electrocardiographic imaging may identify patients with mitral regurgitation at risk for atrial fibrillation and could be used to guide treatment strategies.
Key Words : Atrial Fibrillation, Electrocardiographic Imaging, Inferior Vena Cava.
Ralph J. Damiano, Jr., MD
660 S. Euclid Ave., Campus Box 8234
St. Louis, MO 63110, USA
Patients with mitral regurgitation (MR) commonly develop atrial fibrillation (AF), and AF is present in up to 40% of people referred for mitral valve surgery.1 Worldwide, valvular heart disease is the most common comorbid condition associated with longstanding persistent AF.2 The prevalence of moderate or greater MR in the United States has been estimated to be between 1-2% of the adult population.3 AF in patients with MR has been associated with increased risk of all-cause mortality, stroke, and other cardiovascular morbidity.4 As such, the onset of AF is a Class IIa indication for mitral valve surgery as long as there is at least a 95% likelihood of successful repair and less than 1% predicted risk of mortality.5 Despite its common occurrence and poor prognostic impact in these patients, the mechanism of AF in patients with MR has not been well described, nor have any electrophysiologic predictors of AF been identified. Invasive mapping of patients with MR in small series has shown local electrophysiological characteristics, including conduction slowing and conduction heterogeneity in the left atrial posterior wall (LAPW), that may be a putative substrate for AF in this population.6 The existence of atrial enlargement, conduction heterogeneity and prolonged activation times in severe MR associated with AF has recently been replicated in an chronic animal model of severe MR.7 These changes have been associated with fibrosis and altered histological architecture in experimental heart failure models.8
In patients referred for mitral valve surgery, surgeons have an opportunity to perform concomitant AF surgical ablation. However, there remains little mechanistic information to guide the appropriate ablation strategies and there remains considerable controversy regarding the correct lesion set.9 Most surgical ablation procedures are derived from one of two archetypes: the Cox-Maze procedure and pulmonary vein isolation. The Cox-Maze procedure was developed in 1987 and originally consisted of a mazelike pattern of surgical incisions in both atria.10-13 This pattern of lesions was designed to interrupt macro-reentrant circuits, preventing atrial fibrillation. The Cox-Maze procedure has been modified to be performed using radiofrequency ablation and cryoablation devices, but still requires cardiopulmonary bypass.14 The Cox-Maze procedure has been associated with freedom from atrial fibrillation and anti-arrhythmic drugs over 80% at one year in patients with mitral valve disease in retrospective studies from high-volume centers15, 16 and has been associated with improved long-term survival in a propensity-matched retrospective study.17 Pulmonary vein isolation was first described using endocardial catheters in 1998 and has shown to be effective treatment for AF arising from focal sources in the pulmonary vein ostia.18 Surgical pulmonary vein isolation continues to be used to treat patients with AF referred for mitral valve surgery despite scant evidence of long-term effectiveness of pulmonary vein isolation alone in this population. There have been case series reporting outcomes in patients with AF and valvular heart disease. Gaita and colleagues reported only a 20% freedom from AF and antiarrhythmic drugs at 24 months with PVI alone.19 Additional left atrial lesions in a “U” or “7” configuration were associated with 57% freedom from atrial fibrillation and antiarrhythmic drugs at 24 months. In the CTSNet trial of surgical ablation in patients with mitral valve disease, Gillinov and colleagues reported better results, with 61% freedom from AF at 6 and 12 months with pulmonary vein isolation.20 However, 13% of patients in the ablation group were taking class I or III antiarrhythmic drugs at 12 months. Saint and colleagues showed that omission of even a single lesion from the left atrial posterior wall isolation in the Cox-Maze IV procedure in patients with mitral valve disease was associated with six-fold increased odds of recurrent atrial fibrillation.21
Electrocardiographic imaging (ECGI) was first successfully used for noninvasively mapping human arrhythmias in 2004 and has since been utilized to map normal and abnormal ventricular activation, ventricular repolarization, and atrial fibrillation.22-25 Although noninvasive atrial activation mapping using ECGI in normal sinus rhythm (SR) has been described,23, 26, 27 this technique has not been used to characterize SR atrial activation in patients with structural heart disease. Indeed, most studies in the literature describe mapping a “sinus” or regular supraventricular rhythm as proof of concept to validate EGCI mapping, or focus on atrial flutter, atrial fibrillation or atrial tachycardia.
The present study examined the use of noninvasive ECGI to examine SR atrial activation in patients with moderate or greater MR referred for valve surgery with and without AF. The results in these patients were compared to a cohort of healthy volunteers. The goal of this study was to identify and describe the electrophysiological substrates that lead to AF in patients with MR by comparing subjects with moderate-to-severe MR referred for surgery with and without AF. This was accomplished by analyzing atrial geometry, activation maps, electrogram amplitudes, electrogram deflection counts, and conduction heterogeneity between groups.
The Washington University Biomedical Institutional Review Board approved the protocol for this study. All participants gave written informed consent. This study included data from a total of twenty subjects, who were divided into two groups. Nine subjects with moderate or greater MR and a history of AF underwent ECGI while in sinus rhythm (MR and AF). The second group consisted of eleven subjects with moderate or greater MR and no history of AF (MR alone). All of the subjects had isolated degenerative or calcific mitral valve disease. None of the patients had associated coronary artery disease or other valvular pathology. Subjects who had previous cardiac surgery or catheter ablation were excluded from the study, as were any subjects who were minors, pregnant, prisoners, or undergoing urgent, emergent or emergent salvage procedures.
Electrocardiographic Imaging (ECGI)
Each subject underwent 256-channel body surface potential mapping in SR using a commercially available mapping system (ActiveTwo, BioSemi, Amsterdam, Netherlands). Lead locations and atrial geometry were determined by manual segmentation of computed tomography (CT) scans using commercial software (Amira, FEI, Hillsboro, OR, USA). Analysis was performed using custom scripts in the MATLAB environment (Mathworks, Natick, MA, USA). Data were preprocessed by first eliminating channels in poor contact using a ±5 mV threshold, filtering with a 2nd-order low-pass Butterworth filter with cutoff frequency 25 Hz to reduce noise and a 1st-order high-pass Butterworth filter with cutoff frequency 0.5 Hz to reduce baseline drift. Channels in poor contact lying significantly outside the envelope of the other channels were excluded in a semi-automated fashion. ECGI was used to reconstruct atrial electrograms using the technique previously described by Ramanathan and colleagues.23 The overall sequence of processing is shown in [Figure 1].
Figure 1. Data processing scheme. Data shown are from a normal volunteer imaged previously. A. Usable torso signals identified after low-and high-pass filtering and semi-automated rejection. B. Rejected torso signals. C. Scatterplot showing torso positions of used (blue) and rejected (red) nodes. D. Final torso potentials after P-wave selection and semi-automated rejection. E. Epicardial potentials calculated using inverse reconstruction. F. Epicardial potentials (initial time point) projected onto bi-atrial mesh.
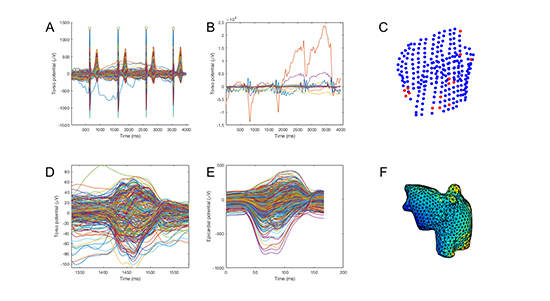
The maximum negative derivative (-dV/dt) method for determining activation times was implemented but was found to produce spatially incoherent results, likely due to far-field signals. Therefore, a discrete wavelet transform was used to detect each deflection in the reconstructed electrogram.24, 28 If a single deflection was identified within the manually defined P-wave window, the maximum negative derivative was used to determine the activation time at that point. If multiple deflections were identified, comparison to neighboring points with solitary deflections within the manually defined P-wave window was used to resolve multiple putative activations.29 Isochronal activation maps were generated. Total, left and right atrial conduction times were manually determined. The total atrial conduction time was the difference between the earliest and latest activation times on the bi-atrial surface. Left and right atrial conduction times were defined as the difference between the earliest and latest activation times on the left and right atrial surfaces, respectively. Activation times were verified by manual examination of electrogram morphology.
Signal amplitude was calculated at each point by finding the difference between the maximum and minimum electrical potential during the P-wave window. Conduction heterogeneity was calculated as the distance-normalized maximum phase difference between each point and its neighbors.30 The heterogeneity index (coefficient of variation, defined as the absolute value of the difference between the 5th and 95th percentiles divided by the median) was calculated.30
Subjects with MR and no history of AF underwent mitral valve repair (n=10) or replacement (n=1) via a minimally invasive right mini-thoracotomy approach (n=10) or median sternotomy (n=1). Ring annuloplasty was performed in each repair, with complex repair techniques performed at the discretion of the operating surgeon (RJD).31 Subjects with AF and MR underwent mitral valve repair with concomitant surgical ablation using the Cox-Maze IV procedure via a right mini-thoracotomy approach (n=8) or median sternotomy (n=1).14, 32
During each operation, a left atrial biopsy was obtained from the margin of the atriotomy in Waterston’s groove. The specimens were fixed with 10% formalin for 24-48 hours, embedded in paraffin and stained with Masson’s trichrome. Bright-field microscopy was performed at 40X magnification. Fibrosis was quantified by identifying blue-stained pixels (blue intensity > 1.2*[red intensity]).33
For continuous variables, Student’s t-test was performed. For categorical variables, Fisher’s exact test was performed. Statistical tests were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P-value < 0.05 was considered significant.
Baseline characteristics and medical history are shown in [Table 1]. Subjects with AF and MR were significantly older than those with MR (p=0.03). There was no significant difference in gender, height or weight. Four of the nine subjects with MR and AF were on antiarrhythmic medication at the time of mapping (2 sotalol, 1 flecainide, 1 amiodarone). Four subjects in the MR alone group were taking a beta-blocker at the time of mapping; the remainder were on no antiarrhythmic drugs. There was no difference in the prevalence of hypertension or in severity of symptoms between subjects with MR with or without AF.
Table 1. Demographics and medical history.
|
MR without AF |
AF and MR |
p |
| n |
11 |
9 |
|
| Atrial Fibrillation Type (%) |
|
|
|
| None |
11 (100.0) |
0 (0.0) |
|
| Paroxysmal |
0 (0.0) |
8 (88.9) |
|
| Persistent |
0 (0.0) |
1 (11.1) |
|
| Age (mean (sd)) |
59 (12) |
70 (9) |
0.028 |
| Male gender (%) |
4 (36) |
5 (56) |
0.684 |
| Weight (kg, mean (sd)) |
73 (16) |
67 (10) |
0.285 |
| Height (cm, mean (sd)) |
168 (14) |
167 (10) |
0.885 |
| Hypertension (%) |
7 (63.6) |
5 (55.6) |
1.000 |
| Antiarrhythmic Drug (%) |
|
|
0.086 |
| Amiodarone and Metoprolol |
0 (0.0) |
1 (11.1) |
|
| Atenolol |
0 (0.0) |
1 (11.1) |
|
| Diltiazem |
0 (0.0) |
1 (11.1) |
|
| Diltiazem and Flecainide |
0 (0.0) |
1 (11.1) |
|
| Metoprolol |
4 (36.4) |
2 (22.2) |
|
| None |
7 (63.6) |
1 (11.1) |
|
| Sotalol |
0 (0.0) |
2 (22.2) |
|
| NYHA Class (%) |
|
|
0.395 |
| I |
2 (18.2) |
2 (22.2) |
|
| II |
7 (63.6) |
4 (44.4) |
|
| III |
0 (0.0) |
2 (22.2) |
|
| IV |
2 (18.2) |
1 (11.1) |
|
AF and MR, mitral regurgitation with history of atrial fibrillation; MR, mitral regurgitation; NYHA, New York Heart Association. Data are presented as mean (standard deviation) except where noted.
Preoperative transthoracic or transesophageal echocardiography was performed for each subject, as determined by the treating physician. There were no significant differences in left atrial size or left ventricular function in subjects with versus without AF [Table 2].
Table 2. Echocardiographic characteristics
|
MR without AF |
AF and MR |
p |
| n |
11 |
9 |
|
| Left ventricular ejection fraction (mean (sd)) |
67.91 (6.99) |
64.44 (7.57) |
0.302 |
| Left atrial diameter (cm, mean (sd)) |
4.54 (1.04) |
4.75 (1.42) |
0.705 |
| Mitral regurgitation grade (%) |
|
|
0.288 |
| Moderate |
2 (18.2) |
4 (44.4) |
|
| Moderate-Severe |
6 (54.5) |
2 (22.2) |
|
| Severe |
3 (27.3) |
3 (33.3) |
|
| Left ventricular end-diastolic diameter (cm, mean (sd)) |
5.04 (0.71) |
4.76 (0.70) |
0.388 |
AF and MR, mitral regurgitation with history of atrial fibrillation; MR, mitral regurgitation. Data are presented as mean (standard deviation) except where noted.
Electrocardiographic Imaging
Subjects with AF and MR had prolonged left atrial conduction times, 110±25 ms compared with MR (85±21, p=0.025), as shown in [Table 3]. There was no significant difference in total or right atrial conduction time or in atrial size. Representative activation maps are shown in [Figure 2], panels A and B.
Table 3. Atrial electrophysiology and anatomy.
|
MR without AF |
AF and MR |
p |
| n |
11 |
9 |
|
| Total atrial conduction time (ms) |
97 (19) |
112 (24) |
0.146 |
| Left atrial conduction time (ms) |
85 (21) |
110 (25) |
0.025 |
| Right atrial conduction time (ms) |
65 (17) |
70 (25) |
0.657 |
| Mean peak-to-peak amplitude (µV) |
138 (68) |
158 (71) |
0.661 |
| Pointwise standard deviation of peak-to-peak amplitude (µV) |
116 (43) |
144 (53) |
0.153 |
| Biatrial surface area (cm2) |
271 (84) |
292 (51) |
0.825 |
| Biatrial volume (mL) |
271 (114) |
316 (94) |
0.750 |
| Number of deflections in P-wave window (%) |
|
|
0.540 |
| 0 |
1.67 (1.35) |
1.33 (1.43) |
0.206 |
| 1 |
58.54 (15.99) |
58.78 (22.32) |
0.520 |
| 2 |
36.22 (13.33) |
33.59 (17.20) |
0.757 |
| ≥ 3 |
3.57 (5.60) |
6.31 (12.78) |
0.362 |
| Pointwise median heterogeneity (ms/mm) |
1.17 (1.09) |
0.85 (0.41) |
0.633 |
| Heterogeneity variation coefficient (index) |
18.29 (10.74) |
14.41 (7.71) |
0.892 |
AF and MR, mitral regurgitation with history of atrial fibrillation; MR, mitral regurgitation. Data are presented as mean (standard deviation) except where noted.
Figure 2. Activation maps: (A) MR (B) AF and MR. Earliest activation is set as time zero; activation times are shown in ms. Amplitude maps: (C) MR (D) AF and MR. AF and MR displays increased variation in electrogram amplitude. Number of deflections: (E) MR (F) AF and MR. MR displays higher numbers of deflections in a consistent location in the left atrial posterior wall; in AF and MR, the pattern is less predictable. Inhomogeneity maps: (G) MR (H) AF and MR. Calculated inhomogeneity delineates lines of conduction slowing or block. The pattern of conduction slowing is much more complex in AF and MR than in MR. IVC, inferior vena cava. LAA, left atrial appendage. LIPV, left inferior pulmonary vein. RAA, right atrial appendage. RSPV, right superior pulmonary vein.
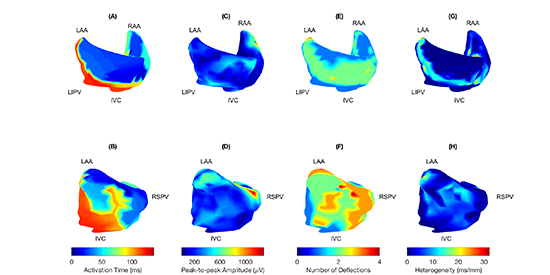
There were no significant differences between groups in electrogram amplitude or in the pointwise standard deviation of electrogram amplitude [Table 3]. Representative amplitude maps are shown in [Figure 2], panels C and D. In patients with MR without AF, a consistent region of multiple deflections was observed in the left atrial posterior wall ([Figure 2], panels E and F). This degree of organization was not present in subjects with AF and MR. There was no difference in conduction heterogeneity (0.97±0.36 ms/mm AF and MR, 1.17±1.02 ms/mm MR without AF, p=0.58). Pointwise heterogeneity maps were useful for identification of areas of conduction block and slowing; examples are shown in [Figure 2], panels G and H. Lines of conduction block or delay in the left atrial posterior wall were seen in 7/9 subjects with AF and MR and in 8/11 subjects with MR without AF, p=1).
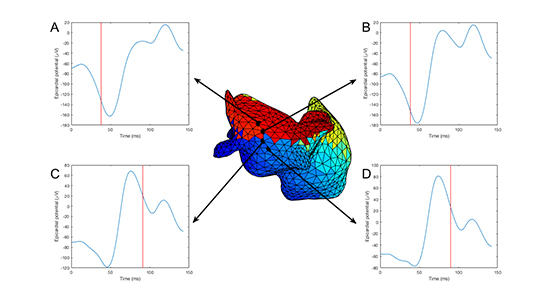
Electrogram progression across a line of block in a previously imaged healthy volunteer is shown in [Figure 3].
Figure 3. Electrogram progression across line of conduction block from a normal volunteer. Four electrograms crossing a line of conduction block in the left atrial posterior wall are shown in blue; activation time is shown by red vertical lines. Electrograms A and B display significantly earlier activation than C and D, and there is a progression of electrogram morphology across the line of block.
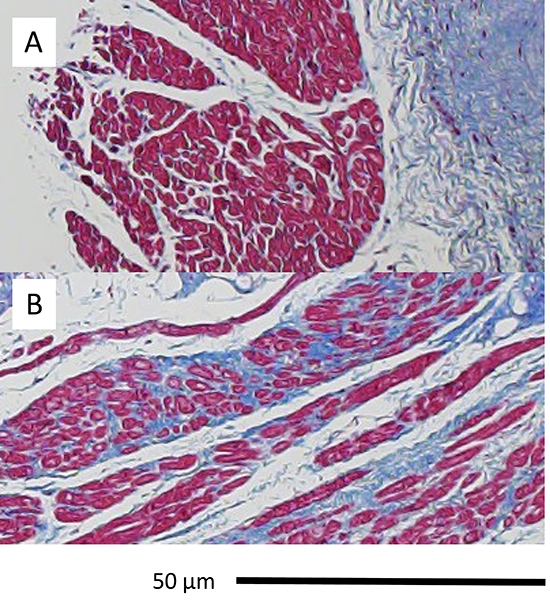
There was no difference in fibrosis in left atrial biopsy specimens from subjects with AF and MR compared with those with MR (19±9% vs. 15±12%, p=0.4). Representative images are shown in [Figure 4].
Figure 4. Histopathology: (A) MR (B) AF and MR. Masson’s trichrome. MR exhibits normal muscle fiber architecture and orientation; AF and MR exhibits interstitial fibrosis.
In this study, noninvasive ECGI was used to characterize atrial activation abnormalities in patients with MR referred for surgery, with and without a history of AF. Subjects with AF were found to have prolonged left atrial conduction times and more complex conduction slowing with unidirectional conduction block when compared with those with MR and no history of AF. These findings on noninvasive ECGI describe a potential substrate for AF in patients with MR. The present study was observational in nature and was intended to generate hypotheses for future research.
This study is unique for several reasons. First, the study population includes only patients with MR in the absence of other structural cardiac disease. Second, the subjects with AF included in this study were able to be rhythm controlled at the time of mapping. Most previous studies using noninvasive mapping have primarily studied subjects with persistent AF. The mapping of patients in SR resulted in much higher signal-to-noise ratio and less subjective interpretation of focal activation patterns than mapping patients in AF due to higher electrogram amplitudes and less complicated conduction patterns. Moreover, to develop a predictive algorithm we needed to identify changes in SR that will be helpful in delineating patients who may develop future AF. Third, the present study used noninvasive panoramic mapping (ECGI) rather than invasive endocardial or epicardial mapping. This would allow for the identification of atrial conduction patterns during the preoperative CT or MRI scans which are normally obtained prior to minimally invasive valve surgery.
Furthermore, the present study demonstrated the utility of electrophysiological metrics besides activation patterns alone for comparing noninvasively obtained maps. Finally, an attempt was made to associate the results with the histopathology of the biopsied left atrium.
Patients with MR are known to be at high risk for developing AF.4 Subjects with AF and MR were found to have complex conduction slowing, primarily in the left atrium, as well as unidirectional lines of conduction slowing or block. These alterations in conduction describe a substrate potentially capable of fibrillation. The overall activation patterns were similar to those identified in previous studies of patients with valvular heart disease using direct contact mapping.34
Most studies of noninvasive mapping of atrial activation have focused on activation and phase mapping of atrial fibrillation.24, 26, 29, 35, 36 Although activation mapping is a first priority in invasive and noninvasive cardiac mapping, quantification of other parameters may provide insights into diagnosis and treatment. Before the advent of echocardiography, 12-lead electrocardiography was commonly used for this purpose. Indeed, research into the effects of pulmonary edema and other physiologic abnormalities on body surface electrocardiograms was an early step which led to the development of ECGI.37
Given the relatively sparse literature regarding appropriate metrics for analysis of SR atrial ECGI data, it was necessary to develop electrophysiological descriptors to allow comparison of maps between subjects. The use of activation times alone results in discarding a majority of the information contained within the signal, which is already attenuated to some degree by the use of digital signal processing to reduce noise. Our use of peak-to-peak amplitude was a rudimentary attempt to derive additional information beyond the activation mapping typically used. Mathematical transformations of the activation map such as the phase map for fibrillation, have been previously described and used for mapping of AF; the use of such techniques to guide ablation is currently controversial.28, 38
Conduction heterogeneity, sometimes referred to as inhomogeneity, is simply a mathematical transformation of a known activation map in SR. The projection of this value onto the atrial surface, combined with review of activation movies, may prove to be useful for identifying lines of conduction block. Activation mapping for identification of hypothesized “driver domains” has been applied for this purpose.39
The fact that differences in atrial fibrosis, measured by trichrome staining, did not reach statistical significance is likely due to a combination of the small sample size, heterogeneity in the subject population and sampling error. A single biopsy of the left atrial wall near the right pulmonary veins is not necessarily diagnostic of atrial fibrosis, which has been shown to be heterogeneously distributed on imaging studies both in our laboratory and by others.40 Biopsies at other sites, such as the left atrial posterior wall, may have produced different results. Our laboratory has previously shown a significant increase in atrial fibrosis in a preclinical model of mitral regurgitation.7 Cardiac magnetic resonance imaging may be helpful in localizing areas of atrial fibrosis, and this could be correlated with ECGI abnormalities.40
The presence of lines of conduction slowing or block in the posterior left atrial wall was unexpected. These areas were associated with areas of multiple deflections in the posterior left atrial wall. Careful inspection shows consistent electrogram morphology and activation time determination across these lines of conduction slowing or block. This phenomenon has been observed by others using direct contact mapping.6, 41 This phenomenon may explain the known arrhythmogenicity of the LAPW and its critical role in interventions for AF.42 It may also explain why pulmonary vein isolation is not effective long-term in this population in our experience.43 Others have shown that artificial lines of conduction block related to rapidly changing electrograms near the interventricular septum have been identified as an artifact of ECGI of the ventricles.29 However, others have described conduction delay and block in the LAPW and, as here, identified similar patterns in epicardial mapping of patients during surgery.44
This study has several limitations. First, the relatively small sample size necessitates that any application of these findings be made with caution. Second, although efforts were made to systematize and automate as much of the ECGI analysis as possible, this technique remains operator-dependent. Specific technical aspects of operating specialized equipment, operator-dependent manual segmentation of the atria on computed tomography slices, the use of custom software, and operator-dependent window selection and reconstruction limit the potential application of these techniques. Further development in automated and reproducible techniques for atrial segmentation and inverse electrocardiography is needed and has been pursued commercially (CardioInsight Technologies, Cleveland, Ohio) to allow broader clinical application. Third, the finding of conduction block in the LAPW can be subject to misinterpretation. Finally, age is a known predictor of AF and was significantly different between subjects with and without AF in this study. The age difference between groups is the strongest confounding factor in this study. Many other characteristics did not differ between groups, including the presence of hypertension, the grade of MR, and left ventricular function. This suggests that the difference in left atrial conduction time and more complex conduction pattern observed is associated with AF, although it is possible that these changes are age-related. However, whether or not the conduction slowing is due to age or the degree of mitral regurgitation still does not invalidate the potential utility of ECGI to help localize the substrate and guide surgical ablation strategy. For instance, if the electrophysiologic derangements are confined to the left atrium, a biatrial approach may be unnecessary. Of course, further studies are needed to investigate this hypothesis.
In summary, we have developed methods for quantitative analysis of SR activation in patients with MR. We have applied these methods to investigate the physiology of atrial activation in patients referred for mitral valve surgery. AF in patients with MR was associated with prolonged left atrial conduction time during SR. Further research into these techniques may allow early identification of patients at risk of AF and noninvasive assessment of treatment effects. Finally, they may provide guidance for more mechanistically directed ablation strategies.