Atrial fibrillation (AF) is the most common arrhythmia in clinical practice affecting an estimated 1% of the U.S. population and over 30 million individuals worldwide [1-4]. AF is usually associated with hypertension, diabetes, aging, obesity, heart failure, ischemic heart disease, and other organic heart diseases. AF is also related to high comorbidities and mortality and severe prognostic implications. Moderate physical exercise, aside from producing a nice, peaceful and well-being sensation, has been associated with a reduced risk of AF [5-7]. However, more strenuous endurance exercise, like the one experiencing elite athletes and marathon runners, seems to increase the risk of AF in healthy athletes without organic heart disease [7-10]. Indeed, it was demonstrated that 5% of moderately trained endurance athletes had asymptomatic AF recorded with implanted cardiac monitor device during a 12-month surveillance period [4]. Since most of the episodes of AF appear at night or after a meal, well-trained athletes usually do not blame exercise or training as the cause of their palpitations [9].
About 20 years ago Karjalainen J, et al. published the first study linking AF with high intensity physical activity [10], and it is estimated that the risk of AF in elite athletes is more than 5 times that in the general population [7]. On the other hand, low physical activity was also found to be a risk factor for the appearance of AF. It seems that both extremes in physical activity intensity tend to promote episodes of AF. There are several changes produced within the heart by endurance exercise. Structural, functional, and electrophysiological alterations occur, namely, atrial enlargement and ventricular hypertrophy practically without systolic function modifications [11]. Ventricular compliance was found to be improved which may provide an improvement in ventricular filling [12]. Another established cardiovascular adaptation is enhanced parasympathetic activity with sinus bradycardia [13]. There is also evidence for an alteration in the intrinsic electrophysiological properties of the heart [14]. Increased occurrence of premature atrial contractions has been reported in elite athletes [15], which may represent a potential trigger for AF development [16]. Premature atrial contractions may act as triggers which may induce sustained AF episodes dependent on the presence of atrial vulnerability [17].
We performed a search in PubMed, SCOPUS and Medline using the MeSH headings or text words atrial fibrillation and exercise, or physical activity or athletes or sports. Conclusions regarding quality and strength of evidence were based on the grading of recommendations, assessment, development, and evaluation system. Since no randomized trials neither interventional studies were available, observational studies were considered acceptable. Long-term prospective cohort studies, case-control or cross-sectional studies were also included in this review. Therefore, we aim to analyze the cardiovascular response to different intensity of endurance exercise and the atrial electrophysiological changes, as well as, the potential pathophysiological mechanisms underlying an increased risk of AF in endurance physical activity.
Possible mechanisms involved in AF development in athletes
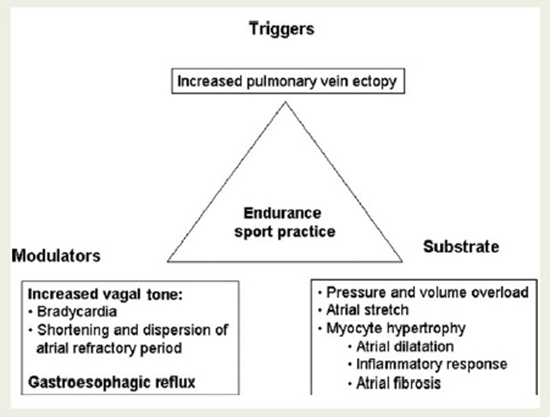
There are several possible mechanisms which may be acting together as etio-pathogenic factors influencing the development of AF in endurance athletes [Figure 1]. It is well known that AF depends on triggers, substrates, modulators, and these factors may be present in association with endurance physical activity. An increased in vagal tone and cholinergic stimulation which occurs in endurance athletes with any vigorous exercise session has been proposed as a plausible mechanism for the genesis of AF in these mentioned subjects. It has been shown in pioneering experimental studies that increased vagal tone shortens the atrial refractory period, and increases dispersion of refractoriness creating appropriate conditions for reentrant circuits to occur [18]. Therefore, the increased vagal tone induced by endurance sport practice may facilitate AF appearance.
In addition, structural changes of the heart mediated by hypertrophy, extensive stiffening of the myocardium with the resultant diastolic dysfunction may cause remodeling and enlargement of the left atrium, pulmonary vein ectopy as triggers and modulators of atrial fibrillation associated with strenuous endurance exercise. Atrial premature contractions, particularly pulmonary vein ectopy, have been shown to be the trigger in most episodes of paroxysmal AF [16]. Atrial premature contractions may be increased as a consequence of physical activity [19]. Therefore, increased ectopy associated to an appropriate atrial substrate may be one of the mechanisms explaining the increased risk for AF associated with sport practice. However, Baldesberger et al. [19] did not find an increased incidence of atrial ectopy in their study in former professional cyclists.
Figure 1. Classical triangle of Coumel suggesting possible etio-pathogenic factors influencing the development of atrial fibrillation in athletes. Reprinted with permission from Mont L, Elosua R, Brugada J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter.
Hemodynamic changes and inflammation
Athletes may experience a chronic increase in atrial pressure due to endurance training. Elevated atrial pressure by itself can lead to atrial dilatation, shortening of atrial refractory periods, and increased incidence of AF. Moreover, atrial inflammation and fibrosis due to repeated exposure to the acute increase in inflammation after prolonged vigorous exertion, was proposed as another underlying possible cause [10]. Indeed, excessive endurance exercise and overtraining can lead to chronic systemic inflammation with inflammatory infiltration as structural atrial changes, and there is a direct relationship between AF and C-reactive protein [20].
There are some hemodynamic changes that occur during exercise. Circulatory flow is 8-fold increased during intense exercise training [21]. The diastolic phase decreases to approximate that of the systolic phase. Hence, the atrioventricular valves are closed for approximately one-half of the cardiac cycle time. This potential for obstruction is attenuated by increases in atrial pressure and contractility during exercise [22]. There is a dilatation of all 4 cardiac chambers due to the intermittent hemodynamic stretch of the myocardium caused by both pressure and volume load during endurance exercise. The right atria and right ventricle may be of greatest clinical relevance given that an excess of cardiac arrhythmia has been demonstrated to originate from these chambers [23].
There are some other aspects related to serum biomarkers in elite athletes. In this context, Stumpf C, et al. [24] studied a total of 25 professional major league soccer players with a mean age 24±4 years and compared them to 20 sedentary controls with a mean age 26±3 years. All subjects underwent physical examination, electrocardiography, echocardiography, exercise testing on a bicycle ergometer, and laboratory analysis of interleukin (IL)-6, tumor necrosis factor (TNF)-a, IL-8, and IL-10. The athletes were divided into two groups according to presence or absence of an early repolarization (ER) pattern. Athletes with an ER pattern showed significantly lower heart rate and an increased E/e´ ratio compared to athletes without an ER pattern. The pro-inflammatory cytokines IL-6, IL-8, TNF-a as well as the anti-inflammatory cytokine IL-10 were significantly elevated in all soccer players. However, athletes with an ER pattern had significantly higher IL-6 plasma levels than athletes without ER pattern. Furthermore, athletes with ''high'' level IL-6 had significantly larger LA volumes than players with ''low'' level IL-6. Therefore, the authors concluded that those athletes with an ER pattern had significantly higher atrial filling pressures, higher LA volume, and higher IL-6 plasma levels.
Although all these factors may contribute to atrial remodeling over time and thus increase the risk of AF in long-term endurance sports, the actual AF development should be corroborated in long-term follow-up. In addition, whether the levels of circulating cytokines have an impact on atrial conduction and arrhythmias over time should also be examined in a larger study with a longer follow-up.
MicroRNA in atrial remodeling
In recent years, microRNAs have been shown to play an important role in in the mechanism of AF development and its pathophysiology by regulating remodeling processes [25]. MicroRNAs are short, single-stranded, and non-coding RNA fragments that bind to the 3' UTR of their target genes leading to inhibition of mRNA translation. Therefore, micro RNAs are post-transcriptional regulators of gene expression. MicroRNAs have been shown to play an important role in atrial remodeling, and especially MiR-1 and MiR-26a are implicated in electrical remodeling by regulating ion channels or calcium homeostasis [26]. On the other hand, MiR-29b, miR-30a and miR-133a are predominantly involved in structural remodeling causing enhanced atrial fibrosis [27]. Another study has shown that endurance sport and aerobic exercise impact on the level of circulating microRNAs [28]. This latter study investigated how the plasma profile of microRNAs and conventional cardiac injury markers like troponin differed, suggesting a potential role for microRNAs as biomarkers for exercise-induced cardiac adaptation. In order to determine the potential value of microRNAs as biomarkers for acute atrial remodeling in athletes, Clauss S, et al. [29] performed the miRathon study, in which they analyzed the plasma profile of 5 microRNAs associated with atrial remodeling in marathon runners. MicroRNAs are important mediators of pro-arrhythmogenic remodeling and have potential value as biomarkers in cardiovascular diseases. In this context, they studied 30 marathon runners who were divided into two age-matched groups depending on the training status: elite (ER, more than 55 km/week, n=15) and non-elite runners (NER, less than 40 km/week, n=15). All runners participated in a 10 week training program before the marathon, and MicroRNA plasma levels were measured at 4 time points: at baseline, after the 10 week training period, immediately after the marathon, and 24h later [29].
In addition, clinical data were obtained including serum chemistry and echocardiography at the four each time point [29]. The authors found that MicroRNA plasma levels were similar in both groups over time with more pronounced changes in elite runners. After the marathon MiR-30a plasma levels increased significantly in both groups. MiR-1 and miR-133a plasma levels also increased but showed significant changes in elite runners only. 24h after the marathon plasma levels returned to baseline. MiR-26a decreased significantly after the marathon in elite runners only and miR-29b showed a non-significant decrease over time in both groups. MicroRNA plasma levels showed a significant correlation with LA diameter in elite runners. However, microRNA plasma levels did not correlate with echocardiographic parameters in non-elite runners [29]. With these results, the authors concluded that microRNAs were differentially expressed in the plasma of marathon runners with more pronounced changes in elite runners. MicroRNA plasma levels correlate with left atrial diameter in elite runners suggesting that circulating microRNAs could potentially serve as biomarkers of atrial remodeling in athletes. The expression levels of these microRNAs observed in Clauss S, et al. are confirmed by other studies that demonstrated a role of these microRNAs in cardiac remodeling [26].
These mentioned results obtained from several studies further support circulating serum microRNAs as potential biomarkers for cardiac remodeling. However, these results should be considered only as hypothesis-generating data and do not prove a direct causal link between circulating serum microRNA levels and the genesis of AF in elite athletes. Long term follow-up clinical studies in endurance physical activity are necessary to provide definitive evidence of this association.
There are some interesting animal experiments that shed light into the mechanisms underlying AF development related to chronic endurance training. Guasch E, et al [30] subjected rats to daily 1-hour treadmill training for 8 or 16 weeks. This mimicked chronic endurance-exercise in athletes. Based on maximum oxygen-uptake, the authors suggested that the 16-weeks treadmill-training regimen in rats corresponds roughly to about 10 years of exercise training in humans. They demonstrated that the rats subjected to chronic exercise were more susceptible to pacing induced AF associated with an enhanced vagal tone, atrial dilatation and increased fibrosis.
These results were similar to findings in humans with long-term endurance training [31]. However, the cessation of exercise reversed AF inducibility in the animal experiments, suggesting a cause-effect relationship between endurance exercise and AF development [30]. It was very interesting to note that although this deconditioning protocol decrease AF inducibility, it did not attenuate atrial dilatation and fibrosis, suggesting that molecular pathways other than structural remodeling also contribute to the AF development in athletes. In this context, Guasch E et al. observed that an enhanced baroreflex and sensitivity to cholinergic stimulation of the G protein-gated K+ channel play central roles in this experimental exercise model [30]. Furthermore, molecular studies suggested that altered mRNA expression levels of several regulators of G-protein signaling proteins may contribute to the increase sensitivity to vagal tone. This study suggested that enhanced vagal activity plays an important role, through increased baro-reflex responsiveness and increased sensitivity to cholinergic stimulation at the level of the atrial cardiomyocytes.
Clinical studies associating AF to physical exercise
AF has been linked to extensive and long-term exercise, as prolonged endurance exercise has shown to increase the incidence and risk of AF. In contrast, light to moderate physical activity is beneficial since it is associated with a decreased risk of AF, and current research indicates a J-shaped association between AF and the broad range of physical activity and exercise [32-49].
Light to moderate physical activity and AF
There seems to be no doubt about the fact that light to moderate physical activity is not associated to higher incidence of AF. Indeed, the Cardiovascular Health Study [32] investigated the association between habitual physical activity and AF among 5,446 adults. The subjects were 65 years of age or older and were followed-up for over a 12-year period. The results showed that, unlike high intensity exercise, light to moderate physical activity is associated with a lower incidence of AF. In addition, a meta-analysis [33] including 95,526 subjects confirmed that regular physical activity is not associated with a higher risk of AF compared with sedentary lifestyle. This result provided additional relevance to the already known beneficial effects of regular exercise on cardiovascular risks.
AF is the most common arrhythmia in middle-aged athletes. Moderate physical exercise performed in a regular basis has been shown to be beneficial for cardiovascular health [41]. Moderate levels of physical activity reduce AF risk as demonstrated in the following study. Physical activity was assessed in 36,513 women at baseline in the Swedish Mammograph Cohort study [42]. Of the total number of women, 2,915 developed AF. The incidence of AF over a median follow-up of 12 years was 15% lower in women exercising over 4 h weekly versus those exercising less than 1 h weekly.
Physical activity was also assessed at baseline in 81,317 women in the Women's Health Initiative Observational Study [43]. In this study 9,792 developed AF. The incidence of AF over an average follow-up of 11.5 years decreased with progressively more physical activity and was 10% lower in women exercising >9 MET task hours per week versus those with no reported weekly exercise. This effect was independent of the body mass index. In this study even the most physically active women, those exercising >15 MET task hours weekly in strenuous physical activity, had a 9% lower rate of AF [43].
In the Women's Health Study, out of 34,759 women, 968 developed AF after a median of 14.4 years [44]. Women exercising 7.5 MET hours weekly had a 14% lower risk of AF, but this was not significant after adjusting for body mass index. On the other hand, AF incidence was 28% lower in the Cardiovascular Health Study with moderate-intensity physical activity [45]. However, those exercising at the highest intensity had a risk of AF not significantly different from the no-exercise group.
Very recently, in the middle of last year, Albrecht M, et al. [34] investigated the association of total and types of physical activity, including walking, cycling, domestic work, gardening and sports, with atrial fibrillation in the Rotterdam Study. The authors studied a prospective population-based cohort which included 7018 participants aged 55 years and older with information on physical activity between the years 1997-2001. They utilized Cox proportional hazards models to examine the association of physical activity with atrial fibrillation risk. Physical activity was categorized in tertiles and the low group was used as reference. They observed during 16.8 years of follow-up that 800 episodes of AF occurred (11.4% of the study population). However, the authors found no association between total physical activity and AF risk in any model. After adjustment for confounders, the hazard ratio and 95% confidence interval for the high physical activity category compared to the low physical activity category was 0.71 (0.80–1.14) for total physical activity [34]. Therefore, they concluded that physical activity is not associated with higher or lower AF risk in older adults. Neither total physical activity nor any of the included physical activity types was associated with AF risk. However, this is not the case with endurance physical exercise in elite athletes.
Endurance physical exercise and AF
The clinical outcome is different with endurance physical exercise in elite athletes. At the beginning of last year, Elliot AD, et al. [35] recruited 99 recreational endurance athletes who were grouped according to lifetime training hours. The athletes underwent evaluation of atrial size, autonomic modulation, and atrial premature contractions. They were grouped by self-reported lifetime training hours: low (<3000 h), medium (3000–6000 h), and high (>6000 h). Left atrial (LA) volume, left ventricular (LV) dimensions, and LV systolic and diastolic function were assessed by echocardiography. A 48-hour ambulatory electrocardiographic monitoring was utilized to determine heart rate, heart rate variability, premature atrial contractions, and premature ventricular contractions. The authors found that LA volume was significantly greater in the High (+5.1 mL/m2, 95% CI: 1.3–8.9) and Medium (+4.2 mL/m2, 95% CI: 0.2–8.1) Groups, compared with the Low Group. LA dilatation was observed in 19.4%, 12.9%, and 0% of the High, Medium, and Low Groups, respectively (P =0.05). They did not observe any differences regarding LV dimensions or function, heart rate variability indices, or premature atrial and ventricular contraction [35]. Therefore, they concluded that increased lifetime training is associated with LA dilatation in the absence of increased vagal parameters or atrial premature contractions in recreational endurance athletes, which may promote incidence of AF in this cohort.
Pathak et al. [40] demonstrated that people with greater exercise capacity with increasing exercise training reduce AF recurrence. These authors evaluated 1,415 consecutive clinic patients with AF. The patients with a body mass index equal to or greater than 27 kg/m2 had a program designed to produce weight loss and increase exercise activity. The patients were divided into low, adequate, and high cardiorespiratory fitness groups on the basis of their baseline exercise performance with an average follow-up of 4 years. Freedom of AF episodes was 12% in the low group, 35% in the adequate group, and 66% in the highest fitness group demonstrating that baseline fitness predicts future AF [40].
This study [40] demonstrated that higher fitness predicts less frequent episodes of AF. However, this is not a randomized controlled clinical trial, but a prospective, observational study, and those individuals adopting an active lifestyle may have made other changes that affected AF risk. Nevertheless, the relationship of exercise to AF is complex, influenced by the intensity and the duration of the physical activity, and seems to have a U-shaped relationship with the greatest levels of physical activity possibly increasing AF incidence. Indeed, the relationship between AF and exercise intensity suggests a curvilinear response with diminishing benefit or even risk with the most intense exercise.
In a similar manner, AF risk increased with the frequency of vigorous exercise in the Physicians' Health Study [46]. Out of 16,921 male participants, 1,661 men developed AF over 12 years of follow-up. Men exercising vigorously 7 days per week had a 20% higher risk. In this regard, a systematic review [47] and a meta-analysis [7] suggested that athletes who engaged in long-term, endurance exercise training have an increased AF incidence. Moreover, among 52,755 cross-country or Nordic skiers who participated in the 90-km races from 1989 to 1998, 919 developed AF before December 2005 [48]. Those athletes who participated in more than 5 races were 30% more likely to develop AF than those who participated in only 1 race.
Another interesting study demonstrated high AF prevalence among long-term, competitive swimmers. Schreiner AD et al. [49] designed a cross-sectional study utilizing survey data to compare the prevalence of AF in swimmers to a general internal medicine population. A multi-national group of swimmers over the age of 60 were surveyed, and a chart review was performed on a random sample of age-matched internal medicine patients. The primary outcome was the diagnosis of AF. Univariate analysis was used for means of proportions of the responses, and a multivariate logistic regression analysis was performed with diagnosis of AF as the dependent variable. Forty-nine swimmers completed surveys and 100 age-matched internal medicine patients underwent chart review. The group of swimmers had 13 cases of AF (26.5 %) compared to 7 (7 %) in the comparison group (p = 0.001). A diagnosis of hypertension or diabetes mellitus was present in 23 (46.9 %) and only 1 (2 %) of the swimmers, respectively, as compared to 72 (72%, p=0.003) and 32 (32%, p<0.001) in the comparison group. Swimming was associated with an odds ratio of 8.739 (95% CI 2.290 to 33.344, p=0.015) [49]. The authors concluded that long-term, competitive swimmers have an increased prevalence of AF compared to internal medicine patients, despite the higher burden of diabetes mellitus and hypertension in the internal medicine group. This suggests that competitive swimming should be included in the list of aerobic activities associated with AF.
Influence of aging and endurance exercise
Myrstad M, et al. [36] investigated the influence of aging and long-term endurance sport practice as a risk factor for AF in elderly men. They compared in a cross sectional study a total of 509 men aged 65–90 years who participated in a long-distance cross-country ski race with a control group of 1768 men aged 65–87 years from the general population. Long-term endurance sport practice was the main exposure. Self-reported AF and covariates were assessed by questionnaires [36]. The authors estimated the risk differences for AF by using a linear regression model. After multivariable adjustment, a history of endurance sport practice gave an added risk for AF of 6.0 percent points (95% confidence interval 0.8–11.1).
Of interest was the finding that light and moderate leisure-time physical activity during the last 12 months reduced the risk with 3.7 and 4.3 percent points, respectively, but the risk differences were not statistically significant. They concluded that this study suggested that elderly men with a history of long-term endurance sport practice have an increased risk of AF compared with elderly men in the general population [36]. This study has the added strength that has a low prevalence of traditional risk factors for AF. Very few of the long-term endurance skiers were smokers, had coronary heart disease or diabetes. The prevalence of hypertension among the athletes was much lower than in the general population.
Risk factors influencing AF development with exercise
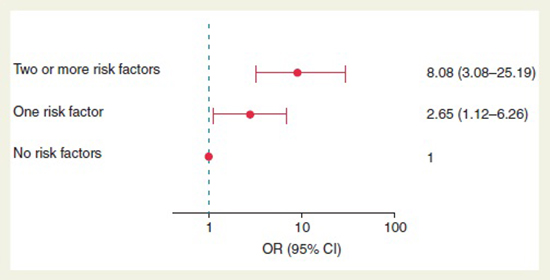
Besides the high-intensity exercise, there are other emerging risk factors that influence the AF development. The more risk factors a patient has, the greater the chances of developing AF [Figure 2]. Calvo N, et al. [37] analyzed several risk factors in a group of 115 patients with lone AF who were compared to 57 age and sex-matched healthy controls in a 2:1 prospective case–control study. The authors obtained and analyze clinical and anthropometric data, transthoracic echocardiography, lifetime physical activity questionnaire, 24-h ambulatory blood pressure monitoring, Berlin questionnaire score, and, in patients at high risk for obstructive sleep apnea syndrome, a polysomnography. Based on conditional logistic regression analysis they found an association of the following four risk factors to a higher AF risk, namely, height [odds ratio (OR) 1.06 (1.01–1.11)], waist circumference (OR 1.06 [1.02–1.11]), obstructive sleep apnea syndrome (OR 5.04 [1.44–17.45]), and 2000 or more hours of cumulative high-intensity endurance training [37]. Of interest, their data indicated a U-shaped association between the extent of high-intensity training and AF risk. The risk of AF increased with an accumulated lifetime endurance sport activity of more than 2000 h compared with sedentary individuals (OR 3.88 [1.55–9.73]). Nevertheless, a history of less than 2000 h of high-intensity training protected against AF when compared with sedentary individuals (OR 0.38 [0.12–0.98]). Therefore, the authors concluded that a history of more than 2000 h of vigorous endurance training, tall stature, abdominal obesity, and obstructive sleep apnea syndrome are frequently encountered risk factors in patients with lone AF. Fewer than 2000 total hours of high-intensity endurance training associates with reduced lone AF risk [37].
Figure 2. Risk of atrial fibrillation in patients according to the presence of risk factors and the impact of physical activity. Reprinted with permission from Calvo N, Ramos P, Montserrat S, et al. Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: a prospective case-control study.
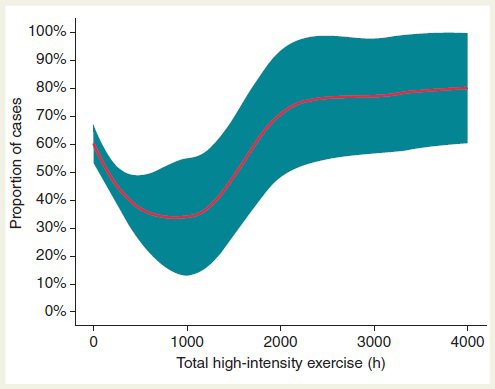
This study described for the first time a U-shaped association between the duration of high-intensity training and the risk of developing lone AF [Figure 3]. Although Calvo N, et al. [37] demonstrated that 2000-h threshold of endurance training better discriminated individuals at risk for exercise-induced AF, the upper limit of safety endurance training has been elusive. In this regard, Elosua R, et al. [38] demonstrated that an accumulated sport practice of more than 1500 h was associated with an increased risk of AF. Data from Drca N, et al. [39] suggested that more than 5 h/week of vigorous intensity exercise at 30 years of age increased AF incidence after 60 years of age. Nevertheless, the dose–response curve of intensity training/AF risk is likely to be continuous showing a high inter-individual variability.
Figure 3. Percentage (95% CI) of participants with lone AF (cases) according to accumulated high-intensity physical exercise (local likelihood regression). Of note, proportion is dependent on cases/control matching in a specific study sample. When considered as a continuous variable, the relationship of lifetime-accumulated high-intensity training to AF risk followed a U-shaped dose–response curve. Reprinted with permission from Calvo N, Ramos P, Montserrat S, et al. Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: a prospective case-control study.
Strain rate and speckle-tracking echocardiography in exercise
In recent years, atrial strain and strain rate analysis by 2-dimensional speckle-tracking echocardiography has emerged as a novel method to evaluate atrial functions. The assessment of atrial function by strain rate and speckle-tracking echocardiography has been used as a predictor of AF recurrence in various clinical situations and in the evaluation of atrial function in male elite athletes [50].
Sanz-de la Garza M, et al [51] designed a study to better understand and characterize the acute atrial response to endurance exercise and the influence of the amount of exercise achieved. They performed 2D ultrasound speckle-tracking strain Echocardiography in 55 healthy adults at baseline and after a 3-stage trail race: a short race (14 km), n=17; a medium race (35 km), n=21; and a long race (56 km), n=17. The authors found that after the race the reservoir function of the right atrium decreased in the medium race group (∆% SRs: -12.5) and further in the long race group (∆% SRs: -15.4), with no changes in the short race group. The contractile function of the right atrium decreased in the long race group (∆% SRa: -9.3), showed no changes in the medium race group (∆% SRa: +0.7), and increased in the short race group (∆% SRa: +14.8).
A similar trend was documented in the reservoir and contractile function of the left atrium but with less pronounced changes [51]. The decrease in the reservoir function of the right atrium after the race correlated with the decrease in the global longitudinal strain (GLS) of the right ventricle (∆% RVGLS vs. RASt and RASRs: +0.44; p<0.05 and +0.41, respectively; p<0.05) [51]. Therefore, the authors concluded that during a trail-running race, an acute exercise-dose dependent impairment in atrial function was observed, mostly in the right atrium, which was related to systolic dysfunction of the right ventricle. The impact on atrial function of long-term endurance training might lead to atrial remodeling, favoring arrhythmia development.
These data from Sanz-de la Garza et al [51] provided some important new insights about post-race cardiac function. They observed a post-race reduction in RV but not LV function. The novel finding was the changes in atrial function which, relative to baseline, augmented after 14 km of running but then progressively reduced over 35 km and 56 km. Dysfunction of the right atrium occurred earlier and was more profound than for the left atrium. It is of interest that the athletes completing the longest race had greater atrial dysfunction despite better pre-race conditioning. It seems that training cannot adequately offer cardiac protection from the significant hemodynamic stress of elite endurance racing.
Data found in previous studies mentioned above indicate a higher incidence of AF among athletes and former competitive athletes compared with the general population. However, this occurs predominately in middle-aged athletes engaged in endurance sport activities over a long time period, which supports the concept that years of endurance training may be necessary before the development of AF [51-53]. The lack of vast prospective studies where the amount of exercise is accurately measured with many years of long-term follow-up does not allow at present time the inception of lifetime hour threshold of sport practice for AF development. Future research should focus on the physiopathology of AF, on the increasing role of inflammation and novel serum biomarkers associated with strenuous physical activity. It is essential to develop effective treatment approaches and to determine to what extent could be useful to utilize anti-inflammatory drug agents.
Declaration of Interest and Funding
None. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.