Atrioesophageal Fistula After Atrial Fibrillation Ablation: A single center series
Houman Khakpour1, Richard J Shemin2, Jay M Lee3, Eric Buch1, Noel G Boyle1, Kalyanam Shivkumar1, Jason S Bradfield1
1UCLA Cardiac Arrhythmia Center, David Geffen School of Medicine at UCLA, Los Angeles, CA.2Division of Cardiac Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA.3Division of Thoracic Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA.
The incidence of atrioesophageal fistula (AEF) after atrial fibrillation catheter ablation is reported to be 0.015%-0.04%, though it is likely underreported due to a number of factors including misdiagnosis. We report our institutional experience with AEF.
>
Patients with confirmed diagnosis of AEF between 2004 and 2016 at our institution were identified (n=5) and their clinical characteristics and outcome were analyzed.
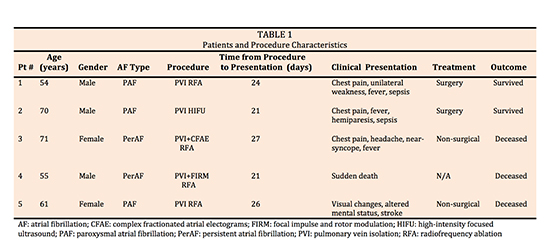
AEF occurred in 5 patients who underwent AF catheter ablation (3 ablated at our institution; 2 transferred from outside hospitals after diagnosis of AEF). Symptoms were chest pain (n=3), fever (n=3), TIA/stroke (n=3), dysphagia (n=1), and headache (n=1). Chest pain was the earliest symptom and occurred 21-24 days post-RFA. One patient had sudden death without preceding symptoms. Findings included leukocytosis (WBC count range of 17200-19,000) and sepsis. Chest CT was obtained in 3 patients and showed air in the left atrium or mediastinum. Three patients had evidence of multifocal stroke on MRI. Three patients died before surgery could be performed. Two patients (40%) underwent emergent surgery which included partial excision of atrial wall, closure with bovine pericardial patch and closure of esophageal lesion. Surgical outcomes were favorable (100% survival).
Chest pain and fever were the early symptoms of AEF and occurred before the neurologic complications. Chest CT was an excellent tool for detection of AEF. All patients who were diagnosed correctly and underwent surgery survived. Early detection is imperative as prompt surgery may improve survival. Health-care community education is the key to ensure early detection and transfer to a qualified surgical center.
Key Words : atrial fibrillation, atrioesophageal fistula, radiofrequency catheter ablation, complications.
Correspondence to: Houman Khakpour, MD
UCLA Cardiac Arrhythmia Center
100 Medical Plaza, Suite 660
Los Angeles, CA
(P) 310-206-2235
(F) 310-825-2092
Hkhakpour@mednet.ucla.edu
Atrioesophageal fistula (AEF) is a rare but serious complication of atrial fibrillation (AF) catheter ablation with associated high mortality rates. The incidence of confirmed AEF after AF catheter ablation is 0.015%-0.04% based on surveys, but this figure is likely underreported due to a number of factors including misdiagnosis and low response rate.[1]–[3] AEF was the second most common cause of mortality after cardiac tamponade, accounting for 16% of fatality related to complications of AF catheter ablation in a large retrospective study. [4] AEF mortality rate has been reported to be 40-100%.[3],[5]–[7] The high mortality rate related to this complication is likely in part due to lack of clinical awareness leading to delayed diagnosis in addition to the complex nature of surgical repair required for treatment.[1],[2],[8] In this study, we report our institutional experience with AEF.
Between 2004 and 2016, all adult patients with confirmed diagnosis of AEF at our institution were identified based on chart review. This included patients who were referred from, diagnosed or initially treated at outside hospitals prior to transfer to our institution. Records of patients were reviewed for demographic and clinical data. Clinical data included index catheter ablation, presenting symptoms and timing, laboratory values, radiographic and pathology results, type of surgical repair, post-operative course and outcomes.
Continuous data are presented as medians and ranges and categorical data are presented by absolute numbers and percentages.
Demographics, Ablation Technique and Clinical Presentation
Five confirmed cases of AEF after catheter ablation were identified between 2004 and 2016. Of these, 3 cases occurred at our institution and the remaining 2 were referred to our center for management of AEF. A total of 1212 AF procedures were done at our institution in that time period. Median age was 61 years (range 54-71). Three were male, 3 had paroxysmal AF and 2 had persistent AF.
Four underwent radiofrequency ablation and 1 had high intensity focus ultrasound (HIFU) ablation (patient 2). All patient had pulmonary vein isolation (PVI). Procedural details for 3 patients with index ablation done at our institution were available (patients 3-5). All procedures were done under general anesthesia. Of these, 2 had ostial PVI (patients 3 and 5) and patient 4 underwent wide area circumferential catheter ablation (WACA). In addition to PVI, patient 4 had Focal Impulse and Rotor Modulation (FIRM) ablation in the left atrial roof, patient 5 had additional posterior roof and lateral mitral isthmus line, and patient 3 underwent ablation of complex fractionated atrial electrograms (CFAEs) in the mid anterior wall, roof, posterior wall inferior to the RIPV and mitral annular region. CFAEs noted adjacent to the esophagus were not ablated. EZ Steer Thermocool irrigated catheter (BioSense Webster Diamond Bar, CA) was used in 2 patients (patient 3 and 4) and an 8 mm Navistar DS non-irrigated catheter ((BioSense Webster Diamond Bar, CA) was used in 1 patient for ablation (patient 5). Power range of 25-40 watts with temperature cut-off of 42°C were employed for irrigated catheters and a power of 50 watts with temperature cut-off of 50°C was used with the non-irrigated catheter. All reported lesions were 30-60 seconds long with loss of local electrogram used as an endpoint. RF applications on the posterior left atrium were limited to 25 watts for patients 3 and 4. Using a single-thermocouple esophageal temperature probe, esophageal luminal temperature (LET) was monitored during ablation in the 3 patients whose index procedure done at our institution. RF applications were stopped when LET reached 38.5°C or there was an increase in LET by >1°C. Maximum reported esophageal temperature did not exceed 39.0 °C. Information on esophageal protective measures were not available for 2 patients who were transferred for management of AEF from outside institutions. 2 out of 5 patients (patients 3 and 4) received prophylactic proton pump inhibitor (PPI) peri-operatively. Devices to displace esophagus were not used for any of the patients.
Patients developed symptoms related to AEF between 21 and 27 days after index ablation procedure (median 24 days). Symptoms included chest pain (n=3), fever (n=3), TIA/stroke symptoms (n=3), dysphagia (n=1), and headache (n=1). Chest pain was the earliest symptom and occurred 21-24 days post-RFA [Figure 1]. When neurologic symptoms were present they included visual disturbance (n=2), hemiparesis (n=2), and hemi-neglect (n=1).
Figure 1. Frequency of Clinical Findings in Five Patients presenting with AEF to our institution
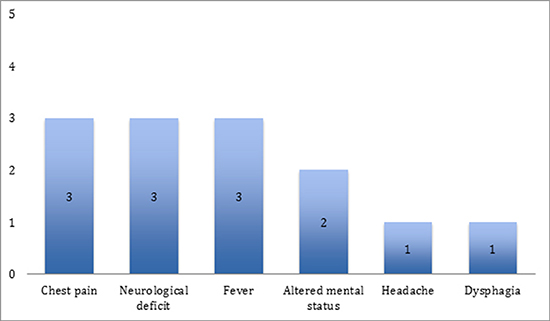
Laboratory and clinical findings included leukocytosis in all of the patients (range 17200-19,000) and sepsis (n=3). Blood cultures were obtained from 3 patients which grew Strep anginosus (patient 1), Strep constellatus (patient 2) and Strep viridans (patient 3).
Intra-operative pathologic specimen cultures were obtained from patients 1 and 2. It revealed Strep anginosus, Lactobacillus, and Candida albicans in one patient (patient 1) and no growth in the other.
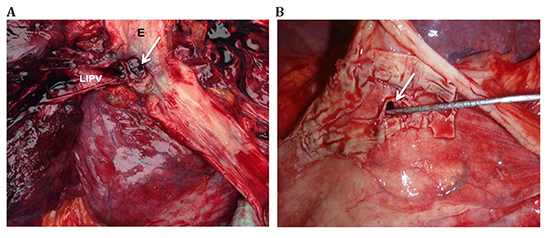
Brain magnetic resonance imaging was performed in 3 patients all of whom had evidence of multifocal embolic stroke (patients 1, 2 and 5). Contrast-enhanced CT scan of chest using intravenous contrast was obtained in 3 patients and showed air in the left atrium or mediastinum (patients 1,2 and 3). The initial chest CT was done on the day of admission for patients 1 and 2 and on the 2nd day for patient 3. CT imaging was repeated on the 2nd day of admission for patient 2 due to an initial non-diagnostic result and a high index of suspicion. Pathology specimens obtained during surgery or autopsy confirmed the diagnosis of AEF in all patients. The level of AEF was noted for 4 of the patients; it was near the RIPV for patient 1, adjacent to LSPV for patient 2, and involving the LIPV for patients 3 and 4. Images of pathologic specimens for 2 of the patients with AEF are shown in [Figure 2]. [Table 1] summarizes the clinical data for each patient.
Figure 2. Pathologic specimens of atrioesophageal fistula (AEF) A: Esophageal (E)-left inferior pulmonary vein (LIPV) fistula (arrow) in patient 4 B: AEF (arrow) near the LIPV with transmural necrosis of LIPV in Patient 3
Surgical Repair of Atrioesophageal Fistula
Two patients underwent emergent surgical repair of the AEF (patient 1 and 2). Both patients presented with chest pain and TIA/stroke symptoms. Chest CT using IV contrast confirmed the diagnosis in both patients.
Surgical repair was performed by a team with a cardiac and a thoracic surgeon. Following median sternotomy and cardiopulmonary bypass, left atriotomy was performed, extending the incision underneath the superior and inferior vena cava. Extensive debridement along the pulmonary veins was done with removal of the left atrial posterior wall and necrotic debris and visualization of the atrioesophageal fistula. A bovine pericardial patch was then sewn to reconstruct the entire posterior wall of the left atrium followed by closure of the atriotomy. Upon discontinuation of cardiopulmonary bypass and closure of median sternotomy, surgical repair of the esophagus was undertaken. Following Esophagogastroduodenoscopy (EGD), the right latissimus dorsi and right 5th intercostal muscles were harvested via a right thoracotomy approach. The distal esophagus was then dissected off the left atrium entirely and the fistula was taken down from the left atrial posterior wall and the descending thoracic aorta. The fistula opening on the esophagus side was closed. A myotomy was performed centered around the esophageal fistula and the right intercostal muscle flap was used to cover the esophageal myotomy. The latissimus dorsi muscle flap was then sutured to the left atrial repair site to ensure complete separation of the posterior left atrial wall from the esophagus.
Table 1. Pathologic specimens of atrioesophageal fistula (AEF) A: Esophageal (E)-left inferior pulmonary vein (LIPV) fistula (arrow) in patient 4 B: AEF (arrow) near the LIPV with transmural necrosis of LIPV in Patient 3
Three patients died before surgery could be performed. In 2 of the 3 patients, death occurred from complications of cerebral embolic events (patients 3 and 5); one died 2 days (patient 3) and the other 5 days (patient 5) after the initial diagnosis of AEF. Non-surgical treatment included broad spectrum antimicrobials and respiratory support with mechanical ventilation. Antimicrobial therapy (for surgical and non-surgical patients) included vancomycin (n=4), piperacillin-tazobactam (n=3), meropenem (n=1) and fluconazole (n=3). One patient had sudden death without preceding symptoms and AEF was diagnosed on autopsy with air embolism as the presumptive cause of death based on autopsy findings. Two patients (40%) underwent successful emergent surgery and survived (100% survival). At one-year follow-up, both patients were alive and free of residual neurologic deficits.
Atrioesophageal fistula is a rare but potentially fatal complication of AF catheter ablation. Symptoms usually develop 3-6 weeks after the index ablation and may be vague, leading to lack of recognition and delayed diagnosis, explaining the high mortality rate.
The pathogenesis of AEF formation is not fully understood. The close anatomic relationship between the esophagus and the left atrial posterior wall likely plays a key role. The esophagus is located in a groove posterior to the left atrium bounded by the thoracic vertebral column and the aorta posteriorly. Thermal injury during ablation is thought to affect the esophageal microvasculature leading to ischemic necrosis and ulceration. Progression from ulceration to AEF formation may be facilitated by esophagitis, adjacent fatty necrosis, gastric hypomotility, and periesophageal vagal plexus injury resulting in lower esophageal sphincter relaxation and acid reflux.[9]–[13] The need for these sub-acute changes to occur could explain the delayed presentation of AEF after ablation.
Several strategies may decrease the risk of AEF formation after catheter ablation. These include use of an esophageal temperature probe for continuous monitoring of esophageal luminal temperature during ablation, real-time visualization of esophagus with barium paste or intracardiac echocardiography (ICE) during the procedure, reduction of power applied to the posterior left atrial wall in the vicinity of the esophagus, prophylactic administration of proton pump inhibitors, mechanical displacement of esophagus or intrapericardial balloon retraction of left atrium, and use of cryoenergy.[14]–[17] Despite these protective measures, risk of AEF is not completely eliminated.[18]–[20]
Clinical presentation and Diagnostic Tools
Patients included in our study presented with a constellation of thoracic, infectious and neurologic findings including chest pain, fever, leukocytosis, sepsis, TIA and stroke between 3-4 weeks after ablation. Chest pain and fever were the leading symptoms of AEF and occurred days to hours before the neurologic complications.
CT scan of chest with intravenous contrast was diagnostic in all the patients who were imaged. The most common findings were air in the left atrium and pneumomediastinum. One patient required a second chest CT one day after the initial imaging due to non-diagnostic initial findings and a high index of suspicion. This imaging modality has been shown to be the most useful tool in multiple prior studies.[7],[21],[22] A high index of suspicion should be guiding image interpretation and in cases of equivocal or non-diagnostic scans, repeat CT imaging may have diagnostic value specially if pre-test probability remains high. Brain MRI was an excellent tool for identifying neurologic sequelae of AEF and when performed, showed multifocal embolic lesions. Diagnostic EGD should not be performed prior to surgical repair due to the inherent risk of worsening air emboli from endoscopic air insufflation required during examination. In the surgical patients, EGD was only performed intraoperatively after the left atrial side of the fistula was repaired in order to identify the site of fistula on the esophageal side to guide operative repair.
There is limited data regarding the prognosis of those patients who survive to undergo surgical repair due to the small number of reported cases, but it appears to be favorable.[8],[18] Based on the data available, the type of repair seems to affect prognosis, with those receiving esophageal stenting having the worst outcome and those undergoing surgery with combined left atrial and esophageal repair having the best result.[18],[21],[23]
In this series, AEF was promptly diagnosed and treated with surgical repair in 2 patients. Both patients survived without any perioperative complications. The surgical technique used was a combined left atrial and esophageal repair. The importance of primary esophageal repair in addition to left atrial repair to minimize postoperative morbidity has been emphasized previously.[21] Interposition of a muscle flap (in our series, intercostal and latissimus dorsi muscle flaps were used) appears beneficial by providing physical separation between the left atrium and esophagus and by allowing the pedicled muscle flap to bring blood supply into a contaminated area.[24]–[26]
Although mixed results have been reported in the literature for the surgical repair of AEF, so far this is the only strategy associated with survival in patients with AEF.[21],[26]–[29] Fatal outcomes after surgical repair may be related to late presentation or delay in diagnosis and intervention. Primary esophageal stenting, while may be successful in treatment of esophageal perforation[30] and even esophagopericardial fistulas[31], has been associated with uniform fatal outcomes for the treatment of confirmed AEF and is therefore not recommended.[18],[21],[32],[33]
Despite preventative measures implemented during AF catheter ablation, esophageal thermal injury may occur for various reasons leading to AEF formation.[18],[34]–[36] Awareness of this complication among the medical community including emergency and primary care physicians is crucial. High index of suspicion is required for prompt diagnosis and treatment. A triad of chest pain, fever, and neurologic symptoms 3-4 weeks after AF ablation is highly concerning for AEF. However, given the low incidence of this complication and an overall lack of familiarity with AF catheter ablation techniques in the ER and primary care community, these symptoms may be attributed to other causes.
All patients with suspected diagnosis of AEF should undergo immediate contrast-enhanced chest CT and be transferred to a hospital equipped with a cardiothoracic surgery facility. Immediate combined left atrial and esophageal repair is the best option based on our experience. Perioperative care in the ICU should include broad-spectrum antimicrobials, early initiation of feeding via jejunostomy tube and ongoing neurologic evaluation and rehabilitation.
Our study has a number of limitations. (1) As expected by the very low prevalence of AEF, our cohort size was small. (2) Complete diagnostic data was lacking on one patient who presented with AEF to an outside facility. (3) Details of procedural techniques and esophageal protective measures during ablation were not available for 2 patients who were transferred to our facility for management of AEF.
Atrioesophageal fistula remains a serious complication of AF catheter ablation despite frequently used esophageal protection strategies. When untreated, the outcome is near-universally fatal. Prompt diagnosis of AEF and urgent referral for surgical repair is imperative as outcomes of early surgical repair are favorable. Health-care community education is the key to ensure early detection and transfer to a qualified surgical center.