Scar homogenization in AF ablation: Evolution and practice
Minglong Chen
The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210029, China.
Laboratory studies, histology studies, image studies and the clinical studies all prove the positive correlation between atrial fibrillation and atrial fibrosis from different perspectives. Atrial fibrosis, by separating myocardial cell coupling, diminishing conduction velocity and promoting anisotropic conduction, produce the substrate to sustain atrial fibrillation (AF). These fibrotic areas can be translated into signal abnormalities (low voltage and complex electrgram), and be depicted by electroanatomic high density map. Ablation targeting these areas after circumferential pulmonary vein produces isolation as the additional substrate modification strategy has proved its beneficial results. However, the unified methodology regarding the scar definition, the mapping rhythm (AF or sinus rhythm) and the modification endpoint is yet to be negotiated. Large-scale clinical trials, long-term follow-up results are needed to prove its contribution to the overall success rate of AF ablation.
Key Words : Atrial fibrillation, Atrial fibrosis, Low voltage zone, Homogenization, Catheter ablation.
Correspondence to: Minglong Chen, MD, Division of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China. Tel: 0086-25-68136965 Fax: 0086-25-6813-6479 E-mail:chenminglong@njmu.edu.cn
Atrial fibrillation (AF) is a common but insidious cardiac arrhythmia. Over half a century passed between the first description of AF on ECG and the development of the most recent catheter ablation treatment, however, the electrophysiological mechanisms underlying AF initiation and maintenance have not been well defined. In the literature, one thing is clear regarding the mechanisms of AF, that is, it requires substrates to perpetuate. These substrates, either electrical or histological, play an important role in AF maintenance. Theoretically, electrical substrate remodeling is often transient and reversible [1] and carries more weight for sustaining paroxysmal AF, while histological remodeling tends to be irreversible and progressive [2] and is the dominant mattress for both paroxysmal and persistent AF perpetuation.
Atrial Fibrosis and Atrial Fibrillation
Any disturbance of the atrial architecture potentially increases the susceptibility to AF [3]-[6] because focal or diffused injury of the atrial myocardium can cause inhomogeneity of atrial repolarization or conduction, thus inducing AF. Such changes (e.g., inflammation, fibrosis, and hypertrophy) occur most commonly in the setting of underlying heart disease associated with hypertension, coronary artery disease, valvular heart disease, cardiomyopathies, and heart failure, which tend to increase left atrium (LA) pressure, cause atrial dilation, and alter wall stress. Similarly, atrial ischemia from coronary artery disease and infiltrative diseases, such as amyloidosis, hemochromatosis, and sarcoidosis, can also promote AF. Additional promoters, including extra-cardiac factors such as hypertension, sleep apnea, obesity, alcohol/medications, and hyperthyroidism—which have pathophysiologic effects on the atrial cellular structure and function—can predispose the atrium to develop AF. Even in patients with lone paroxysmal AF without recognized structural heart disease, atrial biopsies have revealed inflammatory infiltrates consistent with myocarditis and fibrosis [7]. This condition indicates that unexplained atrial myocardial disease underlies lone AF, reflecting a unique type of atrial cardiomyopathy [8]. In fact, all of the above causes, which can be called AF risk factors, will finally give rise to electrical and histological remodeling and therefore promote the genesis of AF.
Atrial cellular impairment and its consequent repairing and remodeling, whatever the etiology, will ultimately result in AF histological substrate. This was confirmed by atrial histological investigations. The atrial histological substrate of AF and its relation to biventricular histological findings were investigated by performing right atrial and biventricular endomyocardial biopsies in patients with paroxysmal lone AF refractory to conventional antiarrhythmic therapy [7]. The atrial biopsies in all cases showed detectable abnormalities, but the histologic findings varied, being compatible with a diagnosis of myocarditis in 66% of patients, with a non-inflammatory cardiomyopathy process in 17% of patients, and with patchy fibrosis in the remaining 17% of patients, likely resulting from myocardial healing caused by a toxic or inflammatory process. In the same population, the histological findings of biventricular biopsies were abnormal only in 25% of patients and confirmed the finding of atrial myocarditis. The results of this study suggest that atrial disease can be independent of and not always secondary to the ventricular disease. Whatever the origin, the inflammation of atrial myocardium is increasingly recognized as a main cause of atrial tachycardia [9], permanent AF [10], [11] and paroxysmal lone AF. Inflammatory markers, such as C-reactive protein and interleukin-6, are elevated in atrial fibrillation patients and may predict the risk of developing future atrial fibrillation [12]. In fact, the final outcome of inflammation is fibrosis. The expression of major extracellular matrix proteins, such as collagen I, collagen III, and fibronectin, confirmed the relationship between AF and fibrosis [13]. When atrial tissue samples from patients with lone AF, AF with mitral valvular disease and sinus rhythm were obtained from the left atrial free wall near the interatrial septum during cardiac surgery, an increase of approximately 100% in collagen I, 50% in collagen III (which was confined to AF with mitral valvular disease), and a slight non-significant increase in fibronectin in left atrial tissue samples of patients with AF were observed [13]. In surgical maze procedures for valvular atrial fibrillation, left atrial tissues in the posterior-wall and right-atrial appendage were obtained from 47 patients [14]. Patients with preoperative AF had larger atrial cell sizes (19.0±5.0 μm vs 13.9±3.5 μm in the left atrium and 17.0±4.8 μm vs 12.3±2.8 μm in the right atrium, p < 0.01, respectively) and a larger amount of intercellular fibrosis (15.8±8.8% vs 6.9±2.4% in the left atrium and 15.2±6.2% vs 6.2±2.9% in the right atrium, p < 0.01, respectively) in both atria compared with those with preoperative sinus rhythm. Additionally, for patients with preoperative AF, those in the unsuccessful maze group had significantly larger atrial cell sizes and a larger amount of intercellular fibrosis in the left atrium compared with those in the successful group. Furthermore, a larger amount of left-atrial intercellular fibrosis compared with the right atrium was observed only in the unsuccessful maze group. Corradi and colleagues [15] evaluated regional left-atrial interstitial remodeling in patients with chronic AF undergoing mitral valve surgery and suggested that the left-atrial free wall around the pulmonary vein ostia was a region characterized by marked interstitial remodeling compared with the left-atrial appendage. A postmortem study [16] provided strong evidence for interstitial atrial fibrosis in patients with AF. These laboratory and clinical studies established a positive correlation between atrial fibrosis and AF from different perspectives.
With atrial fibrosis, the interstitial space between cardiomyocytes is increased due to the accumulation of fibrotic collagen [17], [18], and the electrical side-to-side junctions between muscle bundles are disrupted [19], [20]. The poor tissue coupling, discontinuous propagation and non-uniform anisotropic conduction are the electrophysiological premise for AF.
Detecting the LA scar in AF patients
Electrophysiologically, by separating myocardial cell coupling and diminishing conduction velocity, atrial fibrosis produces lower-amplitude electrograms [21], electrogram fractionation, and conduction heterogeneity and manifests as abnormal signals that can be identified using electroanatomic mapping during sinus rhythm. Thus, the fibrotic areas can be translated into abnormal electrical signals and can be imposed on a three-dimensional map [22], [23]. Two similar studies [22], [23], using a high-density mapping technique, compared the electrophysiological substrate in a different AF population. They found that with the progress of AF, there was a gradual reduction of overall LA mean voltage, prolongation of LA activation time, higher incidence of low voltage zone (LVZ) detection and increased prevalence of complex electrogram. More importantly, in Dr. Lin’s study [23], the definitions of LVZ (bipolar voltage range: 0.1-0.4 mV) and TZ (transitional zone) (bipolar voltage range: 0.4-1.3 mV) were established. Thirteen patients without any cardiovascular risk factors, who underwent left-sided accessory pathway ablation, were supposed to have “normal” LA. In this normal population, 95% of the points had bipolar voltage above 0.38 mV. Therefore, the upper limit cutoff value of the LVZ was defined as 0.4 mV. The complex electrograms during sinus rhythm were defined as any multiphasic electrogram with ≥3 positive or negative distinct peaks and electrogram duration ≥50 ms, suggesting a local conduction delay. When analyzing the distribution of these complex electrograms in the LA body in patients with non-paroxysmal AF, 95% of patients were distributed in the areas with the bipolar voltage <1.32 mV. As such, TZ with bipolar voltages between 0.4 mV and 1.3 mV was defined to facilitate the search for these abnormal electrograms. Theoretically speaking, diseased atria are not only “black and white” with a clear line; therefore, setting LVZ as the profound scar and TZ as the moderate fibrotic area is more reasonable.
The first study addressing the LA scar and its correlation with the catheter ablation outcome was Dr. Verma’s work [24]. The prevalence of the LA scar in AF patients was approximately 6% detected by electroanatomic mapping (bipolar voltage <0.5 mV). AF patients with the LA scar had a significantly higher recurrence rate than those without the LA scar. Image studies, using the technique of late gadolinium-enhanced (LGE)-MRI, demonstrated a significant correlation between LGE-MRI identified enhancement and the burden of fibrosis present on the biopsied tissue [25]. The atrial tissue substrate in the three different AF clinical phenotypes (paroxysmal AF, persistent AF and long-standing persistent AF) was also studied using LGE-MRI. A weak correlation was found between the extent of atrial fibrosis and the presence of persistent AF [26], [27]. There was significant overlap in the degree of fibrosis between patients with different AF phenotypes such that the phenotype did not accurately predict the degree of atrial fibrosis. Moreover, patients with so-called “lone AF”, i.e., AF with no other cardiovascular disease condition, had the same burden of atrial fibrosis as those with non-lone AF [28]. Clinically, Mahnkopf et al. [28] reported that the detection of increased enhancement within the left atrium by delayed-enhanced magnetic resonance imaging was strongly associated with AF recurrence after PVI. Later, the multicenter DECAAF study demonstrated that baseline atrial fibrosis prior to ablation was a major predictor of arrhythmia recurrence. The investigators classified the degree of fibrosis using the MRI image [29]. The MRI data were analyzed at the core laboratory for image quality and for quantification of atrial fibrosis. Based on the degree of detected fibrosis from delayed enhancement MRI, the following 4 stages were defined: stage 1, less than 10% of the atrial wall; stage 2, 10% or greater but less than 20%; stage 3, 20% or greater but less than 30%; and stage 4, 30% or greater. There were 49 patients (18.9%) in stage 1, 107 patients (41.2%) in stage 2, 80 patients (30.8%) in stage 3, and 24 (9.2%) in stage 4. The trial concluded that the overall success rate of AF ablation was independent of ablation strategy, but depends on the extent of atrial fibrosis. Huang et al. [30] elegantly showed that left atrial scarring areas, which were detected using LGE-MRI, correlate well with low voltage areas on electroanatomic maps of the LA. Interestingly, by reviewing the evidence from clinical mapping techniques in combination with imaging and computational modeling, Haissaguerre et al. concluded that AF drivers are located within heterogeneous structural/fibrotic atrial regions [31]. This finding was also described in two other studies, which, by placing the circular mapping catheter at the LVZ during AF, could record the rotational activation [37], [39].
Scar homogenization as the ablation strategy for the treatment of AF
Since the atrial fibrotic areas correlate strongly with AF, catheter ablation targeting these areas as the substrate modification beyond CPVI is a new strategy.
Regional and linear ablation base on the bipolar voltage mapping
In the study of Rolf et al., 178 patients with paroxysmal or persistent AF were included. The confined LVZs were targeted for regional ablation, which aimed to homogenize the diseased LA tissue by radiofrequency ablation [32]. The end point for areal radiofrequency lesions was reached with a significant reduction in local electrograms, defractionation, and loss of capture while stimulating the ablation catheter with high output (10 V; 2 ms). Strategic linear lesions were performed whenever ablative substrate homogenization could not be completed because of potential collateral damage (e.g., septal near the AV-node or posterior close to the esophagus) or when extensive regional ablation might have created critical isthmus sites for potential macro reentrant tachycardias (e.g., near the roof or anterior LA to prevent roof-dependent or perimitral flutter). These strategic linear lesions either connected non-conducting tissues with other anatomic electrical battier structures traversing target LVZs or encircled large LVZs to electrically isolate the diseased tissue from the rest of the healthy atrium. The end point for strategic lesion creation was reached with the confirmation of a complete block (e.g., perimitral conduction) as indicated by (1) reduction of local electrogram amplitude, (2) loss of local capture, (3) confirmation of double potentials on the line and analysis of activation sequence, while stimulating near the linear lesion line. After circumferential PVI with or without substrate modification, burst pacing (10 V; 2 ms) from the proximal coronary sinus was conducted (10-s periods, decreasing cycle lengths from 300 ms until refractoriness in 20-ms steps). Inducible regular atrial tachycardias (AT) were targeted for radiofrequency ablation with AT termination and non-inducibility as the clinical end point. In case of AF inducibility, no further substrate modification was conducted. Success rate at 12 months was 70% in patients with LVZs, and 62% in patients without LVZs. Success rate did not differ significantly in paroxysmal versus patients with persistent AF (69% versus 61%; P=0.28). Similarly, Hans et al. applied box isolation of fibrotic areas [33], [34] in both paroxysmal and non-paroxysmal AF patients and achieved favorable results.
Voltage-guided ablation of the posterior wall beyond CPVI may also improve arrhythmia-free survival [35]. After vein isolation was achieved, posterior wall voltage mapping was performed using a 3-D electroanatomical mapping system during sinus rhythm. The presence of scar was defined as a region that reproducibly demonstrated an area of > 0.5 × 0.5 cm on the posterior wall with a voltage less than 0.5 mV. Posterior wall ablation, if low voltage was found, was preferably performed using a posterior roof line and a floor line completing a posterior wall “box.” Importantly, the borders of the box were intended to encompass the area of low voltage. The low voltage to be targeted without completion of the “box” was allowed if clinically indicated, such as esophageal temperature concerns. Voltage-guided ablation increased 1-year AF/AT free survival in patients compared to standard ablation (80% vs. 57%; P = 0.005).
Another study compared the long-term outcome in patients with paroxysmal AF and severe LA scarring identified by 3-D mapping, in which pulmonary vein antrum isolation (PVAI) only, PVAI and the scar homogenization, or PVAI + ablation of the non-PV triggers was applied [36]. In this particular population, the long-term outcome of scar homogenization plus PVAI was only slightly improved.
Substrate ablation based on the abnormal electrograms during sinus rhythm mapping
According to the voltage mapping, a strategy of selective electrophysiologically guided atrial substrate modification in sinus rhythm (SR) after circumferential pulmonary vein isolation and cavotricuspid isthmus ablation was used [37]. The authors enrolled 86 patients with persistent and long-standing persistent AF. Once SR was restored by cardioversion, high-density bipolar voltage mapping of LA was performed using A-Focus catheter to identify the LVZ (0.1–0.4 mV) and TZ (0.4–1.3 mV). All the electrograms in LVZ were ablated to achieve an absolute bipolar electrogram of <0.1 mV. If SR-AEs were identified in TZ, ablation targeting SR-AE was performed to achieve electric silence or elimination of SR-AE. Additional short linear lesions were placed to transverse potential conducting channels for re-entrant activity between isolation lines or anatomic conduction barriers and LVZs [Figure 1] and [Figure 2]. Among the patients converted to SR, 70% (55/79) had LVZs and TZs with SR-AEs and received additional ablation, whereas in 30% (24/79) of patients without electrophysiological substrate, no further ablation was performed. A total of 78 matched patients who had traditional stepwise ablation strategy were used as the control group. During a follow-up period of >30 months, the Kaplan-Meier estimated probability to maintain SR was 69.8% versus 51.3%. After a single procedure, 3.5% developed post procedural AT in the study group compared with 30% in the control group (P=0.0003). This strategy proposed a more comprehensive substrate modification approach not only targeting the profound fibrotic areas (LVZ) but also addressing the moderate fibrotic areas (TZ). It is analogous to what has been used in conventional pathologic ventricular tachycardia ablation and is supposed to be the combination of both curative (AF) and preventative (AT) strategies. The other reproducible study is from Dr. Yamaguchi’s work [38]. However, modification in transitional areas was not applied.
Figure 1. A case example of sinus rhythm substrates obtained from high-density mapping in the LA after circumferential pulmonary vein isolation; cavo-tricuspid isthmus ablation and cardioversion. Upper panel: low voltage zone (LVZ) defined by 0.1-0.4 mV; Middle panel: Transitional zone (TZ: 0.4-1.4 mV) with complex electrograms; Lower panel: the corresponding lesion placement. Notice that homogenization of the LVZs, elimination of the complex electrograms in TZ region and dechanneling were performed.
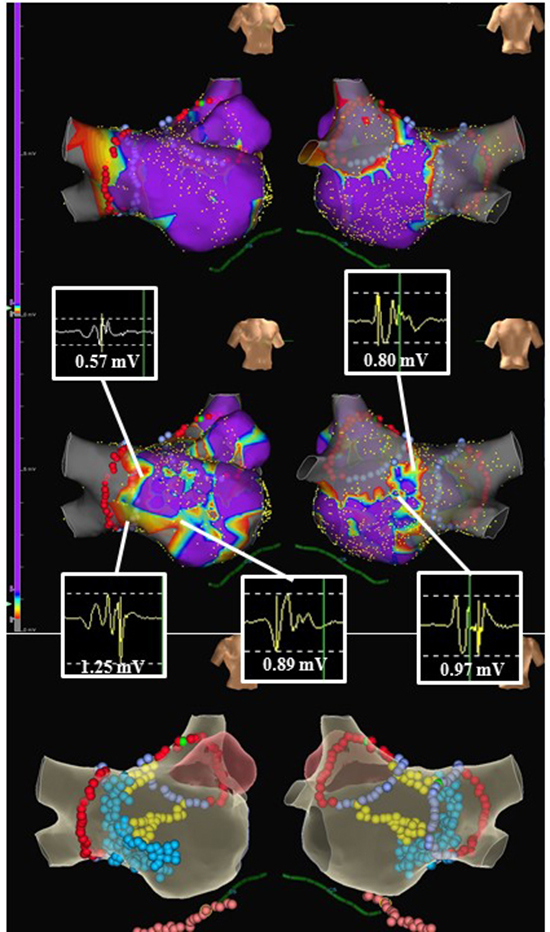
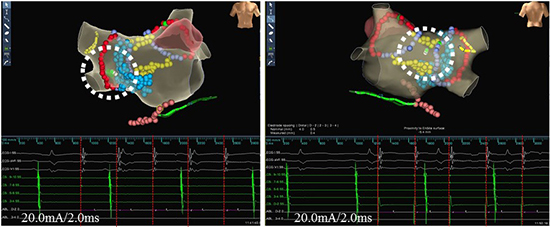
Figure 2. Checking the homogenized or isolated regions using pacing maneuvers. High output pacing from the deflectable ablation catheter placed in the homogenized or isolated regions failed to capture the local myocardium in the same case. Tracings are the surface ECG (I, avL, V1), endocardial recordings of coronary sinus from proximal to distal and the ablation catheter.
Ablation based on electrogram characteristics during AF mapping
During atrial fibrillation, Amir S. Jadidi et al. applied ablation to sites with distinct activation characteristics within/at border zones of LVZ in addition to PVI. This strategy seems to be more effective than the conventional PVI-only strategy for persistent AF [39]. The procedural end point was AF termination and the target areas were defined with electrogram voltage <0.5 mV and electric activity >70% of AF cycle length. In this work, patients presenting in SR were induced by atrial burst pacing from distal or mid-coronary sinus (CS) at a cycle of 250 to 180 ms. If repeat atrial burst stimulation up to 180 ms did not induce AF sustaining for >6 minutes, the patients were considered as non-inducible AF and underwent LA voltage mapping in SR or CS-paced rhythm (at 800 ms pacing cycle length). LA low voltage in sinus or CS paced rhythm was defined as areas with bipolar voltage <1.0 mV. Spontaneous or induced AF underwent initial mapping in the LA and CS, whereas mapping in the right atrium was performed only after unsuccessful LA ablation to reduce procedure length and radiation exposure to patients. Radiofrequency energy was delivered at each low-voltage site displaying the above-mentioned electrogram patterns for 20 s to 40 s. As a result, single procedural arrhythmia freedom at 13 months median follow-up was achieved in 59 of 85 (69%) patients, which was significantly higher than the matched control group (31/66 [47%], P<0.001).
Using the same definition during atrial fibrillation, LVZ-guided substrate modification was performed after PVI in patients with LVZ [40]. A total of 201 patients were enrolled, and more than 80% were non-paroxysmal patients. Larger areas with LVZ were ablated across or along its borders. Isolated LVZ regions were ablated and connected to the closest ablation line or to the mitral annulus. Narrow isthmuses <15 mm were ablated even if local voltage was >0.5 mV, with the exception of the lateral isthmus, which was only ablated if it contained significant LVZ. The authors found after the index procedure, 144 (72%) patients were free from AF at 12 months. With multiple procedures, 148 (74%) patients during a median follow-up of 3.1 years were free from the recurrence [40].
With the evolution of scar homogenization as the additional substrate modification strategy for the treatment of AF, several points remain unclear:
1) Regional variation. Suraj et al. suggested that a bipolar voltage cutoff value of 0.27 mV provided the best discrimination between healthy and unhealthy LA myocardium on DE-CMRI [41]. This value was very close to the lower cutoff value of 0.2 mV that they determined from EAM to identify the scar in the LA-PV junction and LA posterior wall. However, for other LA locations on EAM, a voltage cutoff value of 0.45 mV is more useful in discriminating relatively healthy from scarred LA myocardium. They therefore proposed cutoff values ranging from 0.2 mV to 0.45 mV instead of a single cutoff value, which allows better discrimination of the LA scar when considering regional heterogeneity in LA bipolar voltage distribution. However, in all of the published papers, only one value was used to guide LVZ ablation.
2) Heterogeneity of the electrode spacing of the mapping catheter. Most of the above-mentioned studies used the bipolar voltage of 0.5 mV to define the scar, yet the electrode spacing of the circular mapping catheter and the ablation catheter was obviously different.
3) Variation of the mapping rhythm. Most of the scar definition was based on the signals taken during sinus rhythm; however, the definition of a scar in some other studies was based on the mapping results during AF or CS pacing. Although there was a linear voltage correlation between sinus rhythm and AF, suggesting that a similar extent of left atrial fibrotic substrate could be identified on electroanatomical voltage mapping by adjusting the voltage cutoff [42], the matching value during different rhythm has not been settled.
Methodology of scar homogenization
1) Scar homogenization, scar isolation, scar-based linear lesions and dechanneling are the most common substrate modification strategies applied in recent studies. However, a unified end-point is lacking.
2) The fibrotic substrate is heterogeneous and cannot only be defined as healthy (normal) or unhealthy (scar).
3) Whether the right atrium scar mapping and ablation can further improve the overall success rate is unclear.
Image studies and electroanatomic mapping studies of the AF population have provided a better understanding of the human atrial substrate that maintains AF. This has led to the concept of fibrosis-guided substrate modification. This strategy, though very promising, currently lacks sufficient supportive evidence from the larger population and long-term follow-up results. More clinical evidence should be accumulated, randomized clinical trials should be evidences conducted, and, more importantly, the methodology should be unified. In the near future, the 3-D mapping system, working towards high density and high-resolution map, will help to make scar homogenization more accurate.