Comparison of Phrenic Nerve Injury during Atrial Fibrillation Ablation between Different Modalities, Pathophysiology and Management
Valay Parikh MD, Marcin Kowalski MD MBA FHRS
Division of Electrophysiology, Department of Cardiology, Staten Island University Hospital, Northwell Health System, NY.
Atrial fibrillation ablation has emerged as an effective tool in the management of symptomatic atrial fibrillation. Currently, the electrophysiologists are striving to maximize the success while minimizing complications. Phrenic nerve injury (PNI) is one of the concerning complications, especially in cases of cryoballoon ablation. Due to anatomical proximity to atrial tissue, phrenic nerve is particularly susceptible to injury. With evolving monitoring techniques it is now possible to minimize the likelihood of a permanent PNI. However, the challenge remains to detect PNI at the earliest and to avoid further damage to the nerve. In this review, we discuss pertinent anatomical principles, techniques to avoid PNI and management in cases where PNI is encountered.
Key Words : Phrenic Nerve, Atrial Fibrillation, Ablation.
Correspondence to: Dr. Marcin Kowalski,
Director, Division of Cardiac Electrophysiology,
Department of Cardiology
Staten Island University Hospital, Northwell Health System,
475 Seaview Ave,
Staten Island, NY 10305.
Ablation to achieve pulmonary vein isolation (PVI) has been highly successful in the management of atrial fibrillation (AF).1 However, it is also associated with a variety of complications including phrenic nerve injury (PNI). The anatomic course of the phrenic nerves near the pulmonary veins (PV) predisposes them to injury during the ablation. PNI has been reported as a rare complication during radiofrequency ablation (RFA) especially when a wide area circumferential ablation is deployed.2-4 However, with the emergence of cryoballoon ablation (CBA) as an effective tool to achieve PVI, PNI has become more common with the rate ranging from 8% to 11% in some studies.5-7 There is no reliable method to predict phrenic nerve injury prior to procedure; however, implementing vigorous monitoring of the nerve function can assure early detection and prevent permanent PNI. Hence, it is imperative for electrophysiologists and laboratory staffs to be familiar with and recognize this injury at the earliest to mitigate the damage.
Phrenic nerves (PN) originates from the 3rd to 5th cervical nerves and provides the only motor supply to the diaphragm as well as sensory supply to the central tendon, mediastinal pleura and pericardium. Right PN (RPN) descends vertically from its origin and continues along the right anterolateral surface of the superior vena cava (SVC). Descending down the anterolateral wall of SVC, it turns posteriorly as it approaches the superior cavoatrial junction and follows in close proximity to the right sided PVs. It is separated by only the pericardium at the anterolateral junction between the SVC and the right atrium.8 At this level, distance between the atrial/SVC tissue and RPN on an average is between 0 to 2.3 mm. The closer relationship of the RPN to the Right Superior Pulmonary Vein (RSPV) makes it more susceptible to injury during ablation of the RSPV then during ablation of the Right Inferior Pulmonary Vein (RIPV). Similarly, left phrenic nerve runs anterior to left sided pulmonary veins and are at a danger of PNI during ablation. Furthermore, it can course close to either apex or roof of the mouth of left atrial appendage (LAA), depending upon the anatomical variation, making ablation performed in the vicinity of LAA more complicated.9 Both the phrenic nerves run along with pericardiophrenic artery and vein form a neurovascular bundle in the fibrous pericardium. It is hypothesized that the injury to the phrenic nerve might be due to damage or infarction of the pericardiophrenic artery during cryo ablation.
Definition and Epidemiology
The stages of phrenic nerve damage can be categorized as a) early PN injury defined as any detection of PN damage prior to detectable decrease in diaphragmatic excursion, b) PN injury defined as any decrease in diaphragmatic excursion resolved prior to end of the procedure, c) PN palsy as diaphragmatic paralysis confirmed by exhalation and inhalation x-ray with elevated diaphragm lasting less than 3 months and d) PN paralysis as any PN palsy lasting more than 3 months. ‘Early PNI’ and ‘PNI’ are often described collectively as ‘transient phrenic nerve injury’.10
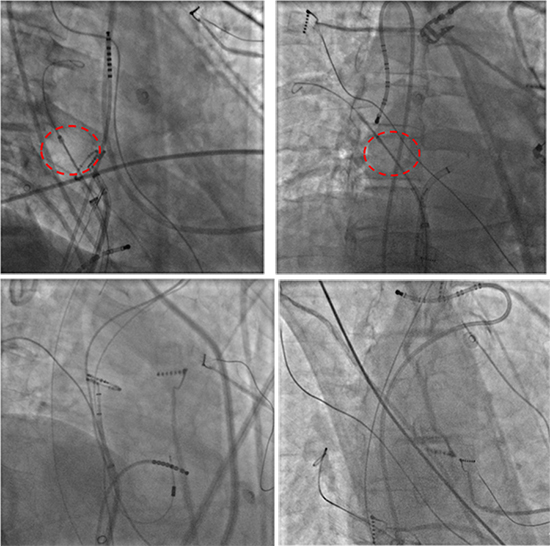
Figure 1 Position of different catheters in the superior vena cava to facilitate capture of the phrenic nerve. (A) Deflectable octapolar catheter (Bisosense Webster Diamond Bar, CA) located on the later wall of the SVC. Notice that the phrenic nerve is captured above the cryoballoon. (B) Deflectable decapolar catheter (Bisosense Webster Diamond Bar, CA) prolapsed into the SVC. Notice the retroflexed curve for better stability. (C) LASSO Circular Mapping Catheter (Bisosense Webster Diamond Bar, CA) and a more distal passion of the decapolar catheter advanced distal in the SVC (D) for stable phrenic nerve capture. Modified with permission from Kowalski M: Prevention of phrenic nerve palsy during cryoballoon ablation for atrial fibrillation. In Ngai-Yin Chan eds: The Practice of Catheter Cryoablation for Cardiac Arrhythmias. Hoboken:Wiley Blackwell, 2014, pp. 67-81
PNI is a more common complication associated with cryoballoon ablation than with radiofrequency ablation.5,11,12 The earlier cryoballoon studies with traditional monitoring approach have reported high incidence of PNI, ranging from 4% -11%.5,6,13-15 However, subsequent studies using novel monitoring methods in adjunction to standard pace-mapping monitoring have reported a sharp drop in this incidence to the order of 1%. 16-20 PNI is more common during the ablation of the RSPV than the RIPV due to the closer proximity of the PN to the RSPV than to the RIPV. Injury to left PN is a possibility during ablation involving LAA and rarely, with ablation of the left superior pulmonary vein (LSPV).21,22
Histo-Pathological Changes
Detailed examination of the histo-pathological changes occurring with PNI has provided a better understanding of the underlying mechanisms. These changes differ based upon the type of energy used and are well-characterized in pre-clinical studies.23,24 In a pre-clinical RF study, Bunch et al demonstrated a graded response to temperature rise and duration of RF applications on PN function.23 In this study, PNI was reversible at a temperature of 47 ± 3oC after 38 ± 32 seconds, while it resulted in a permanent injury with additional RF application of 92 ± 83 seconds at a temperature of 51 ± 6oC. These dose-dependent responses were also reflective in histo-pathological changes. Permanent PNI showed manifestations of acute thermal injury such as edema, coagulation and, irreversible chromatin and cytoplasmic content damage. While transient PNI showed no signs of any nerve damage. In contrast to RF, wallerian degeneration of nerve with focal injury to large axonal neurons is the primary finding seen with cryo induced PNI.24 This is usually distributed in subperineural fashion. Just like RF, degree of damage due to cryo-application was proportionate to the temperature changes and overall amount of energy delivered at a time. In a study done by Andrade et al, lower and earlier nadir temperature was associated with worse outcomes. In this study, nadir ablation temperature, −56.4 ± 7.3 oC with standard monitoring and −52.7 ± 9.8 oC with compound motor action potential (CMAP) monitoring were associated with higher likelihood of PNI. Also, CMAP monitoring, with 30% cutoff to interrupt cryo-application, demonstrated lesser degree of nerve damage as compared to the traditional monitoring method. These findings correspond to observations made in recent clinical studies. It also brings home the point that earlier the termination milder the damage.
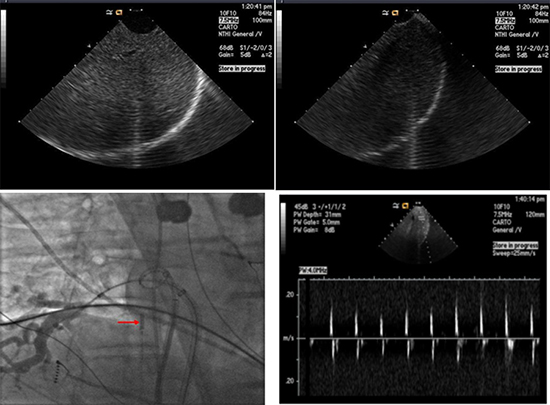
Figure 2 Intracardiac echocardiographic images of the diaphragm and the liver during phrenic nerve pacing showing the diaphragm (A) relaxing and (B) contracting. (C) Fluoroscopy image showing position of intra-cardiac echocardiography catheter (arrow) at the level of diaphragm. (Reproduced with permission from Lakhani M, Saiful F, Bekheit S, Kowalski M. Use of Intracardiac Echocardiography for Early Detection of Phrenic Nerve Injury During Cryoballoon Pulmonary Vein Isolation. J Cardiovasc Electrophysiol 2012 April 11) (D) Pulse Doppler of the liver motion during phrenic nerve pacing. If the entire liver cannot be easily visualized, a pulse wave Doppler can be placed on the liver to observe the liver exertions as a Doppler waveform. A decrease in Doppler amplitude can indicate PN injury. Notice the change in the amplitude of the velocity due to respiratory variation
In both of these studies, PNI occurred relatively early during the ablation and was associated with higher temperature gradient. This can be partly due to better catheter contact which may result in relatively low blood flow and higher energy delivery; or it may be just a reflection of the damage caused by the energy delivery. Furthermore, PNI is more likely to happen at subsequent energy deliveries. This may be due to the fact that nerve tissue may retain some of the effect from the previous energy deliveries, which can be potentiated by subsequent energy application.
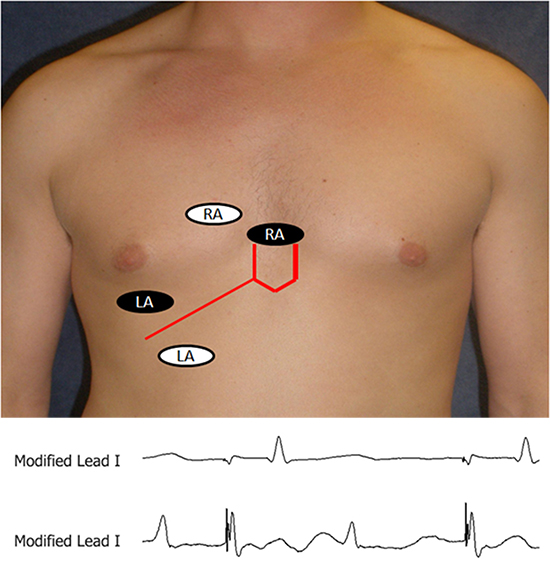
Figure 3 Configuration of surface electrodes to record of diaphragmatic compound motor action potential on modified lead I in an obese patient. The right arm (RA black oval) surface electrode placed 5 cm above the xiphoid and the left arm (LA black oval) surface electrode is placed 16 cm from the xiphoid along the costal margin and corresponding CMAP recordings on modified lead showing low amplitude signal during PN capture (top recording). In some instances the leads require to be modified because of patient’s body habitus by moving the leads to the right and more separated (white ovals) to obtain the optimal CMAP amplitude (lower recording). (Reproduced with permission from Lakhani M, Saiful F, Parikh V, Goyal N, Bekheit S, Kowalski M. Recordings of diaphragmatic electromyograms during cryoballoon ablation for atrial fibrillation accurately predict phrenic nerve injury. Heart Rhythm. 2014 Mar;11(3):369-74. PubMed PMID: 24252287. Epub 2013/11/21. eng. )
The best management strategy for PNI is to vigorously monitor phrenic nerve function and stop the ablation at the first sign of PNI. There is no reliable method of predicting PNI prior to procedure. Pre-procedural imaging of the right pericardiophrenic artery using computerized tomographic angiography can reliably locate the right phrenic nerve.25-27 This technique may identify anatomy more vulnerable to phrenic nerve injury using balloon based ablation systems, although, their clinical utility is limited at this time primarily due to increase radiation, cost and contrast. This method is also of limited value in patients with significant dysrhythmias due to higher gating errors. Another helpful method to predicting PNI prior to energy delivery is to localize a vertical line crossing the distal SVC PN pacing catheter and the lateral edge of the cryoballoon in an anterior-posterior view. If the PN pacing catheter crosses the lateral edge of the balloon, negative predictive value for PNI is 98%13Fig 1.
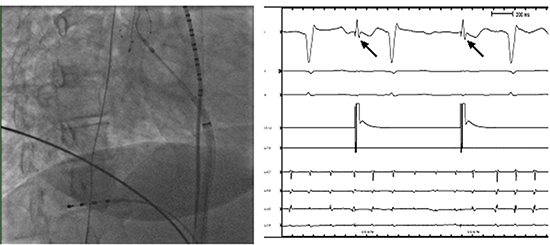
Figure 5 Diaphragmatic compound motor action potential (CMAP). A) Catheter position for phrenic CMAP recording. B) Surface ECG lead V1 and a diaphragmatic CMAP tracing obtained by a quadripolar catheter in the right hepatic vein. The quadripolar catheter is positioned in a hepatic vein to record phrenic CMAP. A multipolar catheter is placed in the SVC to pace the right PN.
It is now recognized that closer the phrenic nerve to the atrial tissue higher its susceptibility to temperature changes and higher the likelihood of PNI. In cases of CBA, the balloon inflation alters PV geometry and may displace PN. In a canine study conducted by Okumura et al, the inflated balloon at PV orifice extended PV diameter by 5.6 ± 3.7 mm anteriorly and 2.7 ± 3.5 mm posteriorly to the original PV diameter.28 This prominent distortion displaced the phrenic nerve closer to the atrial tissue by 4.3 ± 2.9 mm. The degree of this distortion can further be amplified if the balloon is inflated inside the PVs to minimize peri-balloon leak. This is a prime reason why PNI were common with 23- mm diameter cryoballoons. A 23-mm balloon is more likely to be advanced deeper inside the PV as compared to a 28-mm balloon. To overcome this issue of inflation inside the veins, the depth of the balloon can be assessed by a ‘pullback’ method. According to this method, the balloon is pulled back slightly after complete occlusion is demonstrated. By pulling back enough to observe a slight dye leak, the distortion can be minimized without compromising ablation efficacy.6 Similarly, a wide area circumferential ablation (WACA) approach can reduce the incidence of PNI in RFA by avoiding proximity to PN.4
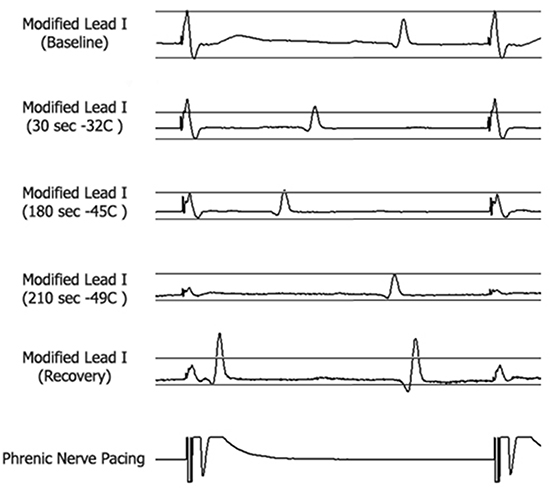
Figure 4 Recordings of the diaphragmatic CMAP during pacing from the multipolar catheter at a CL of 1000 ms located in the SVC. . The CMAP amplitude was measured at baseline (top panel) and was continuously monitored during RSPV ablation by placing horizontal calipers on the screen of a recording system at the level of 35% below the baseline CMAP amplitude (Panel 2). Note attenuation of CMAP amplitude by > 35% from baseline amplitude at 180 seconds of cryoballoon application (Panel 3). At the time of phrenic nerve palsy (210 sec) the CMAP amplitude was a fraction of the baseline CMAP amplitude (Panel 4). The amplitude of CMAP, despite some recovery two minutes after discontinuation of cryo-energy, did not return to baseline value (Panel 5). (Reproduced with permission from Lakhani M, Saiful F, Parikh V, Goyal N, Bekheit S, Kowalski M. Recordings of diaphragmatic electromyograms during cryoballoon ablation for atrial fibrillation accurately predict phrenic nerve injury. Heart Rhythm. 2014 Mar;11(3):369-74. PubMed PMID: 24252287. Epub 2013/11/21. eng.)
During the Procedure- Monitoring the Function
At present, pace-mapping of phrenic nerve throughout the ‘concerning’ part of the ablation remains the only existing method to monitor phrenic nerve function. Currently, there are no practical strategies available to measure phrenic nerve potentials without pacing the phrenic nerve.
PN function is monitored during ablation by advancing a pacing catheter to an optimal site of phrenic nerve capture and pacing at a reasonably high output to ensure capture. It is crucial that this pacing catheter is always placed above the level of ablation, capturing the phrenic nerve at twice the capture threshold. A high current strength can potentially overcome early nerve injury and conceal incipient damage to the nerve. The optimal site for PN capture during ablation of right sided pulmonary veins is the anterolateral portion of the SVC, near the atrial-SVC junction because, at that location, the PN is separated from the SVC wall only by pericardium or near the junction of the SVC. However, Ghosh et al demonstrated that pacing from right subclavian vein may provide better stability and lower threshold values for PN capture.29 This method can particularly be valuable in cases where catheter stability is an issue. Similarly, left phrenic nerve can be captured from left subclavian vein and monitored if needed. Any catheter can be used to capture PN. In our experience, we found a deflectable decapolar catheter to be most useful. Stability and consistent phrenic nerve capture is extremely important. Lack of good contact and loss of capture due to catheter movement may mimic PNI and cause unwarranted cessation of the procedure. Electroanatomic mapping can be useful to tag the capture site and reposition the catheter in case of movement of the catheter. The pacing should be done at cycle lengths that range from 1500 ms to 1000 ms. A slower rate can delay the detection of PNI, while a rapid rate can prematurely fatigue the diaphragm.30 It is imperative that paralytics are avoided during ablation. If they have been administered during the induction of general anesthesia, it is important to begin pulmonary vein isolation only after allowing sufficient time to wean off their paralytic effect or using reversal agents such as neostigamine.
Strategies to Monitor Phrenic Nerve Function
Currently, there is no reliable method that predicts PNI prior to the procedure and it can occur despite of the most compulsive monitoring. In addition to standard monitoring methods, recently, novel techniques are reported. Table 1
Table 1. Pros and cons of various PN monitoring strategies
| Method |
Description |
Advantages |
Disadvantages |
| Fluoroscopy |
Direct visualization of diaphragmatic motion |
• Sensitive method for monitoring diaphragmatic motion |
• Additional radiation exposure to the patient and the operator
• Does not predict early PN injury |
| Palpation |
Palpation of diaphragmatic excursion |
• Reliable and practical method for monitoring diaphragmatic motion
• Abundant data |
• Requires additional staff member
• The palpable strength of diaphragmatic excursion may vary with respiration |
| Electromyography |
Recording of diaphragmatic compound motor action potential (CMAP) by two standard surface electrodes positioned across the diaphragm or by advancing a quadripolar catheter in the right- hepatic vein during PN pacing |
• Earliest detection of PN injury
• Simple reliable and easily applicable
• The only technique that predicts PN injury
|
• CMAP signals might be susceptible to respiratory variations.
• The baseline amplitude must be adequate
• Affected by paralytic agents |
| Auditory cardiotocograph |
Decrescendo pitch on fetal heart monitor (placed across patient’s chest that can detect diaphragmatic contractions) |
• Auditory cue to the operator
• May portend PN injury prior to palsy |
• Extra equipment difficult to record in obese patients |
| Intracardiac echocardiogram (ICE) |
Direct visualization of diaphragmatic excursion |
• minimal radiation exposure to the patient and the operator |
•Requires additional venous access and intra-cardiac ultrasound |
| Modified pediatric blood pressure cuff |
Measurement of pressure changes associated with diaphragmatic movement |
• Objective evidence
• Obviates need for fluoroscopy and additional staff |
• Needs a verification studies including larger patient sample
• Special blood pressure cuff needed |
| Venous Waveform |
Analyzing changes in femoral venous waveform |
• Minimal changes needed to actual procedure
• Obviates need for fluoroscopy and additional staff |
• Needs verification studies including larger patient sample |
Comparison of different strategies for monitoring phrenic nerve palsy during cryoballoon ablation. (Modified with permission from Kowalski M: Prevention of phrenic nerve palsy during cryoballoon ablation for atrial fibrillation. In Ngai-Yin Chan eds: The Practice of Catheter Cryoablation for Cardiac Arrhythmias. Hoboken:Wiley Blackwell, 2014, pp. 67-81)
Continuous or intermittent fluoroscopy of the right hemi-diaphragm during PN pacing can accurately diagnose a diminished diaphragmatic excursion; however, it is the least optimal method as it exposes the patient and operator to additional radiation. Palpation of the strength of diaphragmatic excursion during PN pacing, below the costal margin is the most commonly employed method of monitoring PN function. Diaphragmatic contractions during PN pacing are sensed by placing the hand over the right diaphragm and below the costal margin and palpating every excursion. Weakening of the diaphragmatic contraction can indicate PN injury. This method is easily applicable but the subjectivity associated with the measurement and respiratory variations in the diaphragmatic contraction strength can be mistaken as PNI. Although these methods are considered as a gold standard and practiced widely, they are not the earliest to detect PNI.
Intracardiac echocardiography (ICE) may be utilized to continuously visualize the motion of the liver capsule and indirectly image the contraction of the diaphragm during phrenic nerve pacing.31 The transducer is positioned at the level of the diaphragm and pointed at the liver Fig 2. The decrease in intensity of liver movement from the diaphragmatic excursion can be easily observed and can correlate with phrenic nerve palsy. An external fetal heart Doppler monitor placed at the costal margin can also be used to monitor PN function.10 A decrease in diaphragmatic contraction can be recognized by change in the pitch. The fetal heart monitor can provide an auditory cue to the physician and staff of impending PNI.
The methods of monitoring PN function mentioned above rely on reduction of in the mechanical function of the diaphragm. Novel techniques utilizing diaphragmatic compound motor action potential (CMAP) may provide earlier warning of PN injury.16-18 CMAPs can be successfully recoded on modified lead I by placing a standard surface right arm EKG electrode 5 cm above the xiphoid and a left arm EKG electrode 16 cm along the right costal margin Fig 3. The CMAP amplitude is measured from peak to peak with each PN capture. The largest amplitude signal at baseline is compared to the real-time CMAP amplitude during the ablation. Studies have shown that a decrease in CMAP amplitude by 35% from baseline predicted and prevented PNI Fig 4.16 The CMAP amplitude can be continuously monitored during ablation by placing a horizontal caliper on the screen of a recording system at the level of 35% below the baseline CMAP amplitude. The average time interval from CMAP amplitude decrease of 35% to palpable PNI was 59 seconds (range 30-110 seconds) in published studies, although transient PNI may occur in less than 10 seconds.
An alternative method to this is advancing a quadripolar catheter in the right hepatic vein and recording phrenic CMAP amplitude during PN pacing Fig 5.19,32 The ablation is discontinued if the observed CMAP amplitude decreased by ≥30% from baseline. The study evaluating this method found no reported cases of PNI, including the patients in which ablation was discontinued early, due to decrease in CMAP amplitude. Monitoring PN function using a modified lead I is simple and easily applicable. The surface electrodes may be subject to CMAP amplitude variations with respiratory movements and body habitus. It may be difficult to obtain CMAP recordings in obese patients. Adjusting the electrode to a more superior location may help obtain a better signal in these patients, as the viscera push the diaphragm superiorly when the patient is supine. Monitoring CMAP amplitude by advancing a quadripolar catheter in the hepatic vein is an excellent alternative method especially in patients in whom the recorded CMAP amplitude is less than 0.2 mV. Hence the operator may initially attempt to record CMAP amplitude using modified lead I. If the amplitude is < 0.2 mV or shows significant respiratory variation, a catheter can be advanced into the hepatic vein to record CMAP amplitude. The recorded CMAP amplitude by either method can also be easily displayed directly on the electrophysiology workstation and followed during ablation Figure 5.
Recently, few other novel methods have been proposed on similar principles. One of them was utilizing diaphragmatic contraction was described by MacVeigh et al.33 They measured strength of diaphragmatic contractions with the help of modified neonatal blood pressure cuff tied to right coastal margin. In a small group of patients, they demonstrated that change in the pressure measured with the cuff corresponded to changes seen in diaphragmatic contraction strength. Another recently proposed method have used femoral vein pressure waveform to accurately measure phrenic nerve function.29 With a cut-off of 50% reduction observed in baseline peak to peak venous waveform amplitude to discontinuation of ablation, identification of PNI by this method preceded by a mean of 28s to traditional palpation method. This observation is very similar to other novel methods stressing the point that the commonly employed method of palpation is slower to identify PNI. Although these methods appear promising, they are yet to be confirmed and reproduced in larger sample of patients.
Recently, a series of publications have demonstrated that monitoring with 2 methods (Traditional palpation and a novel method) have decreased the incidence of PNI associated with cryoballoon ablation significantly.16,17,19,34 This may be partly due to the earliest detection of PNI with novel methods. However, further studies are needed to decide if a novel method can be employed as a stand-alone method.
After the Phrenic Nerve Injury
Early detection of PNI and immediate termination of ablation remains the cornerstone of the prevention of PN palsy. It is imperative to be cautious and continuously monitor the PN function by pacing the phrenic nerve above the level of ablation. Ablation should be interrupted immediately at the first sign of PNI. In cases of cryoballoon ablation, immediate balloon deflation by ‘double-tap’ the stop button on the console would prevent persistent PNI.15 Since the decrease in the CMAP amplitude is the earliest sign of detectable injury to the nerve and is simple and easily measurable, it should be used as the primary technique for monitoring in conjunction with one or two other methods. These methods include either palpation of the diaphragmatic excursion or movement of liver visualized on ICE or a fetal heart monitor.
Table 2. Recommendations to Prevent Phrenic Nerve Injury during cryoballoon ablation for atrial fibrillation
| Avoid long-acting paralytics during ablation |
| Consider performing inhalation and exhalation chest x-ray in patients with previous history of CABG to evaluate for injury to left PN |
| Inflate the balloon outside the PV and maintain the balloon as antrally as possible to prevent anatomic distortion of the PV orifice. WACA approach is recommended in RFA. |
| Monitor the rate of temperature changes. A steep descent (colder than -40 degrees at 30 seconds) may indicate distal location of the balloon in CBA. |
| Rigorously monitor PN function by pacing the phrenic nerve from the SVC above the cryoballoon |
| The stimulation of the PN should be carried out at twice the pacing threshold and PN function should be monitored for both RSPV and RIPV. |
| Simultaneously employ diaphragmatic CMAP amplitude and one or two additional techniques to monitor phrenic nerve function |
| Terminate ablation immediately if there is suspicion of PNI. Immediate deflation of the balloon can be initiated by pressing the emergency deflation button twice on the console |
| Always maintain the balloon as antral as possible to the PV os. |
| Utilize the “pull back” method to assess the depth of the balloon inside the PV |
Modified with permission Kowalski M: Prevention of phrenic nerve palsy during cryoballoon ablation for atrial fibrillation. In Ngai-Yin Chan eds: The Practice of Catheter Cryoablation for Cardiac Arrhythmias. Hoboken:Wiley Blackwell, 2014, pp. 67-81
If the phrenic nerve function returns within few minutes, a cautious attempt at ablation can be made again with a more antral position of the balloon. In cases of CBA, the wire/catheter used to engage the vein can be manipulated to select a different branch of the vein so that the balloon-vein angle is modified. If PN function does not return in a reasonable time, then the ablation may be completed using radiofrequency with a wide antral circumferential ablation, which is shown to be safer.4 Additionally, the ablation catheter can be paced at high outputs (10 mA at 2 ms) at putative ablation sites, to discern phrenic capture. Capture of phrenic nerve with high output indicates higher risk of PN injury at that particular site and RF ablation should be performed more antrally. An inhalation and exhalation chest x-ray evaluating the motion of the right diaphragm can assess resolution of PN injury after the procedure.
Clinical Course and Prognosis
The presentation of PNI can range from asymptomatic to cough, dyspnea, and severe respiratory complications. The severity of presentation primarily depends upon the degree of PNI and underlying lung capacity.2,35-37 Clinical presentation can vary broadly ranging from asymptomatic to severe respiratory dysfunction, however, majority of them are asymptomatic or mildly symptomatic. Nonetheless, considering potentiality of significant morbidity, PNI should not be discarded as a benign condition and a cautious follow-up is warranted.
Prognosis of PNI is reasonably good. Most of the cases are self-resolving between 3 to 12 months, although, rarely it can be permanent.2,35 Most of the PNI recover within a year. The recovery largely depends upon the degree of damage and time needed for nerve to regenerate. Therefore, it is essential to be vigilant and stop the ablation instantaneously on losing phrenic nerve capture.
Phrenic nerve injury is a rare, but a potentially dangerous, complication. PNI is higher with balloon catheter based ablations as compared to radio-frequency ablation. However, novel adjunctive techniques are bridging this gap and making these procedures safer in this regards. Irrespective, it is essential for the EP team to be cognizant of this complication, avoid inadvertent injury and detect it at the earliest.